Abstract
Purpose
To evaluate the impact of the addition of quantitative apparent diffusion coefficient (ADC) data into the diagnostic performance of the Prostate Imaging Reporting and Data System version 2 (PI-RADSv2) scoring system to predict clinically significant prostate cancer (CSPCa).
Methods
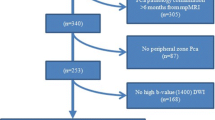
We retrospectively included 91 consecutive patients who underwent prostate multiparametric magnetic resonance imaging (mp-MRI) and histopathological evaluation. Mp-MRI images were reported by the PI-RADSv2 scoring system and patients were divided into groups considering the likelihood of CSPCa. ADC value and ratio were obtained. Findings were correlated with histopathological data.
Results
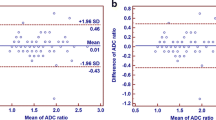
CSPCa was found in 41.8% of cases (n = 38). PI-RADSv2 score 3–5 yielded a sensitivity of 97.4% (95% confidence intervals 86.5–99.5), a specificity of 50.9% (37.9–63.9), and AUC of 0.74 (0.67–0.81) to predict CSPCa. ADC value < 750 µm2/s and an ADC ratio < 0.62 were the most accurate thresholds for differentiation of CSPCa, with AUC of 0.81 and 0.76, respectively. Combined PI-RADSv2 score 3–5 and ADC value < 750 µm2/s yielded a specificity of 84.9 (72.9–92.2), sensitivity of 70.3 (54.2–82.5), and AUC of 0.77 (0.68–0.86). Combined PI-RADSv2 score 3–5 and ADC ratio < 0.62 yielded a specificity of 86.5 (74.7–93.3), sensitivity of was 64.9 (48.8–78.2), and AUC of 0.75 (0.66–0.84).
Conclusion
Quantitative ADC data might not be beneficial to be used routinely in mp-MR imaging as criteria to detect clinically significant lesions due to the reduced sensitivity. Instead, when prostate lesions present a PI-RADSv2 score ≥ 3, additional quantitative ADC criteria can be helpful to increase the PI-RADS score specificity.



Similar content being viewed by others
References
Quon JS, Moosavi B, Khanna M, Flood TA, Lim CS, Schieda N (2015) False positive and false negative diagnoses of prostate cancer at multi-parametric prostate MRI in active surveillance. Insights Imaging 6(4):449–463
Stewart RW, Lizama S, Peairs K, Sateia HF, Choi Y (2017) Screening for prostate cancer. Semin Oncol 44(1):47–56
Purysko AS, Rosenkrantz AB, Barentsz JO, Weinreb JC, Macura KJ (2016) PI-RADS version 2: a pictorial update. Radiographics 36(5):1354–1372
Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A et al (2015) Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 68(6):1045–1053
Kim CK, Park BK, Kim B (2010) Diffusion-weighted MRI at 3 T for the evaluation of prostate cancer. Am J Roentgenol 194(6):1461–1469
Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C et al (2011) Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology 259(3):775–784
Thörmer G, Otto J, Reiss-Zimmermann M, Seiwerts M, Moche M, Garnov N et al (2012) Diagnostic value of ADC in patients with prostate cancer: influence of the choice of b values. Eur Radiol 22(8):1820–1828
Peng Y, Jiang Y, Yang C, Brown JB, Antic T, Sethi I et al (2013) Quantitative analysis of multiparametric prostate MR images: differentiation between prostate cancer and normal tissue and correlation with Gleason score—a computer-aided diagnosis development study. Radiology 267(3):787–796
Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA et al (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461
Ma T, Yang S, Jing H, Cong L, Cao Z, Liu Z et al (2018) Apparent diffusion coefficients in prostate cancer: correlation with molecular markers Ki-67, HIF-1α and VEGF. NMR Biomed 31(3):e3884
Park SY, Kim CK, Park BK, Lee HM, Lee KS (2011) Prediction of biochemical recurrence following radical prostatectomy in men with prostate cancer by diffusion-weighted magnetic resonance imaging: initial results. Eur Radiol 21(5):1111–1118
Giles SL, Morgan VA, Riches SF, Thomas K, Parker C, deSouza NM (2011) Apparent diffusion coefficient as a predictive biomarker of prostate cancer progression: value of fast and slow diffusion components. Am J Roentgenol 196(3):586–591
Haffner J, Potiron E, Bouyé S, Puech P, Leroy X, Lemaitre L et al (2009) Peripheral zone prostate cancers: location and intraprostatic patterns of spread at histopathology. Prostate 69(3):276–282
Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ et al (2016) PI-RADS prostate imaging–reporting and data system: 2015, version 2. Eur Urol 69(1):16–40
Rosenkrantz AB, Ginocchio LA, Cornfeld D, Froemming AT, Gupta RT, Turkbey B et al (2016) Interobserver reproducibility of the PI-RADS version 2 lexicon: a multicenter study of six experienced prostate radiologists. Radiology 280(3):793–804
Greer MD, Brown AM, Shih JH, Summers RM, Marko J, Law YM et al (2017) Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging 45(2):579–585
Rosenkrantz AB, Oto A, Turkbey B, Westphalen AC (2016) Prostate Imaging Reporting and Data System (PI-RADS), version 2: a critical look. Am J Roentgenol 206(6):1179–1183
Woo S, Kim SY, Lee J, Kim SH, Cho JY (2016) PI-RADS version 2 for prediction of pathological downgrading after radical prostatectomy: a preliminary study in patients with biopsy-proven Gleason Score 7 (3+ 4) prostate cancer. Eur Radiol 26(10):3580–3587
Mehralivand S, Bednarova S, Shih JH, Mertan FV, Gaur S, Merino MJ et al (2017) Prospective evaluation of PI-RADS™ version 2 using the international society of urological pathology prostate cancer grade group system. J Urol 198(3):583–590
Rosenkrantz AB, Babb JS, Taneja SS, Ream JM (2016) Proposed adjustments to PI-RADS version 2 decision rules: impact on prostate cancer detection. Radiology 283(1):119–129
Mertan FV, Berman R, Szajek K, Pinto PA, Choyke PL, Turkbey B (2016) Evaluating the role of mpMRI in prostate cancer assessment. Expert Rev Med Devices 13(2):129–141
Gaur S, Harmon S, Rosenblum L, Greer MD, Mehralivand S, Coskun M et al (2018) Can apparent diffusion coefficient values assist PI-RADS Version 2 DWI scoring? A correlation study using the PI-RADSv2 and international society of urological pathology systems. Am J Roentgenol 211:W1–W9
Park SY, Shin SJ, Jung DC, Cho NH, Choi YD, Rha KH, Hong SJ, Oh YT (2017) PI-RADS version 2: quantitative analysis aids reliable interpretation of diffusion-weighted imaging for prostate cancer. Eur Radiol 27(7):2776–2783
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40(2):244–252
Carroll PR, Parsons JK, Andriole G, Bahnson RR, Castle EP, Catalona WJ, Dahl DM, Davis JW, Epstein JI, Etzioni RB, Farrington T (2016) NCCN guidelines insights: prostate cancer early detection, version 2.2016. J Natl Compr Canc Netw 14(5):509–519
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T et al (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65(1):124–137
Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW et al (2011) Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol 29(16):2185–2190
Ahmed HU, Bosaily AE, Brown LC, Gabe R, Kaplan R, Parmar MK, Collaco-Moraes Y, Ward K, Hindley RG, Freeman A, Kirkham AP (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389(10071):815–822
Baldisserotto M, Neto EJ, Carvalhal G, de Toledo AF, de Almeida CM, Cairoli CE et al (2016) Validation of PI-RADS vol 2 for prostate cancer diagnosis with MRI at 3T using an external phased-array coil. J Magn Reson Imaging 44(5):1354–1359
Zhao C, Gao G, Fang D, Li F, Yang X, Wang H et al (2016) The efficiency of multiparametric magnetic resonance imaging (mpMRI) using PI-RADS Version 2 in the diagnosis of clinically significant prostate cancer. Clin Imaging 40(5):885–888
Henderson DR, de Souza NM, Thomas K, Riches SF, Morgan VA, Sohaib SA et al (2016) Nine-year follow-up for a study of diffusion-weighted magnetic resonance imaging in a prospective prostate cancer active surveillance cohort. Eur Urol 69(6):1028–1033
Jordan EJ, Fiske C, Zagoria R, Westphalen AC (2018) PI-RADS v2 and ADC values: is there room for improvement? Abdom Radiol (NY) 43(11):3109–3116
Lebovici A, Sfrangeu SA, Feier D, Caraiani C, Lucan C, Suciu M et al (2014) Evaluation of the normal-to-diseased apparent diffusion coefficient ratio as an indicator of prostate cancer aggressiveness. BMC Med Imaging 14(1):15
Barrett T, Priest AN, Lawrence EM, Goldman DA, Warren AY, Gnanapragasam VJ et al (2015) Ratio of tumor to normal prostate tissue apparent diffusion coefficient as a method for quantifying DWI of the prostate. Am J Roentgenol 205(6):W585–W593
Litjens GJ, Hambrock T, Hulsbergen-van de Kaa C, Barentsz JO, Huisman HJ (2012) Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology 265(1):260–266
Acknowledgements
This study was conducted in accordance with the amended Declaration of Helsinki and with the approval of the local Institutional Review Board.
Author information
Authors and Affiliations
Contributions
MOM: Project development, Data collection and management, Data analysis, Manuscript writing/editing. DHHR: Project development, Data collection and management, Data analysis, Manuscript writing/editing. JC: Data collection and management, Data analysis. FSS: Data collection and management, Data analysis. AA: Data collection and management, Data analysis. JAPN: Data collection and management, Data analysis. GC: Data collection and management, Data analysis. EJDN: Data collection and management, Data analysis. MZ: Data management, Data analysis, Manuscript writing/editing. MB: Project development, Data collection and management, Data analysis, Manuscript writing/editing. BH: Project development, Data collection and management, Data analysis, Manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any conflict of interest to express, including financial or personal relationships that could inappropriately influence his or her actions.
Ethical approval
This study has gained ethical approval from the local research review board (PUCRS/HSL Committee, Brazil, IRB00008131). All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moraes, M.O., Roman, D.H.H., Copetti, J. et al. Effects of the addition of quantitative apparent diffusion coefficient data on the diagnostic performance of the PI-RADS v2 scoring system to detect clinically significant prostate cancer. World J Urol 38, 981–991 (2020). https://doi.org/10.1007/s00345-019-02827-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02827-2