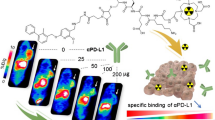
Abstract
Purpose
Due to tumor heterogeneity, immunohistochemistry (IHC) showed poor accuracy in detecting the expression of programmed cell death ligand-1 (PD-L1) in patients. Positron emission tomography (PET) imaging is considered as a non-invasive technique to detect PD-L1 expression at the molecular level visually, real-timely and quantitatively. This study aimed to develop novel peptide-based radiotracers [68Ga]/[18F]AlF-NOTA-IMB for accurately detecting the PD-L1 expression and guiding the cancer immunotherapy.
Methods
NOTA-IMB was prepared by connecting 2,2′-(7-(2-((2,5-dioxopyrrolidin-1-yl)oxy)- 2-oxoethyl)-1,4,7-triazonane-1,4-diyl) diacetic acid (NOTA-NHS) with PD-L1-targeted peptide IMB, and further radiolabeled with 68Ga or 18F-AlF. In vitro binding assay was conducted to confirm the ability of [68Ga]/[18F]AlF-NOTA-IMB to detect the expression of PD-L1. In vivo PET imaging of [68Ga]NOTA-IMB and [18F]AlF-NOTA-IMB in different tumor-bearing mice was performed, and dynamic changes of PD-L1 expression level induced by immunotherapy were monitored. Radioautography, western blotting, immunofluorescence staining and biodistribution analysis were carried out to further evaluate the specificity of radiotracers and efficacy of PD-L1 antibody immunotherapy.
Results
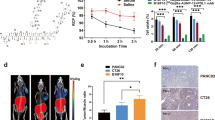
[68Ga]NOTA-IMB and [18F]AlF-NOTA-IMB were both successfully prepared with high radiochemical yield (> 95% and > 60%, n = 5) and radiochemical purity (> 95% and > 98%, n = 5). Both tracers showed high affinity to human and murine PD-L1 with the dissociation constant (Kd) of 1.00 ± 0.16/1.09 ± 0.21 nM (A375-hPD-L1, n = 3) and 1.56 ± 0.58/1.21 ± 0.39 nM (MC38, n = 3), respectively. In vitro cell uptake assay revealed that both tracers can specifically bind to PD-L1 positive cancer cells A375-hPD-L1 and MC38 (5.45 ± 0.33/3.65 ± 0.15%AD and 5.87 ± 0.27/2.78 ± 0.08%AD at 120 min, n = 3). In vivo PET imaging and biodistribution analysis showed that the tracer [68Ga]NOTA-IMB and [18F]AlF-NOTA-IMB had high accumulation in A375-hPD-L1 and MC38 tumors, but low uptake in A375 tumor. Treatment of Atezolizumab induced dynamic changes of PD-L1 expression in MC38 tumor-bearing mice, and the tumor uptake of [68Ga]NOTA-IMB decreased from 3.30 ± 0.29%ID/mL to 1.58 ± 0.29%ID/mL (n = 3, P = 0.026) after five treatments. Similarly, the tumor uptake of [18F]AlF-NOTA-IMB decreased from 3.27 ± 0.63%ID/mL to 0.89 ± 0.18%ID/mL (n = 3, P = 0.0004) after five treatments. However, no significant difference was observed in the tumor uptake before and after PBS treatment. Biodistribution, radioautography, western blotting and immunofluorescence staining analysis further demonstrated that the expression level of PD-L1 in tumor-bearing mice treated with Atezolizumab significantly reduced about 3 times and correlated well with the PET imaging results.
Conclusion
[68Ga]NOTA-IMB and [18F]AlF-NOTA-IMB were successfully prepared for PET imaging the PD-L1 expression noninvasively and quantitatively. Dynamic changes of PD-L1 expression caused by immunotherapy can be sensitively detected by both tracers. Hence, the peptide-based radiotracers [68Ga]NOTA-IMB and [18F]AlF-NOTA-IMB can be applied for accurately detecting the PD-L1 expression in different tumors and monitoring the efficacy of immunotherapy.
Graphical Abstract
Two novel peptide-based radiotracers [68Ga]NOTA-IMB and [18F]AlF-NOTA-IMB were developed as promising agents for evaluating the immunotherapy effect by monitoring the changes of PD-L1 expression in real time.










Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21.
Zhu S, Zhang T, Zheng L, Liu H, Song W, Liu D, et al. Combination strategies to maximize the benefits of cancer immunotherapy. J Hematol Oncol. 2021;14:156.
Monk BJ, Enomoto T, Kast WM, McCormack M, Tan DSP, Wu X, et al. Integration of immunotherapy into treatment of cervical cancer: recent data and ongoing trials. Cancer Treat Rev. 2022;106:102385.
Vesely MD, Zhang T, Chen L. Resistance mechanisms to anti-PD cancer immunotherapy. Annu Rev Immunol. 2022;40:45–74.
Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD-1 pathway. Sci Adv. 2020;6:eabd2712.
Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–70.
Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of high tumor mutation burden in non-small cell lung cancers with increased immune infiltration and improved clinical outcomes of PD-L1 blockade across PD-L1 expression levels. JAMA Oncol. 2022;8:1160–8.
Chen X, Wang K, Jiang S, Sun H, Che X, Zhang M, et al. eEF2K promotes PD-L1 stabilization through inactivating GSK3β in melanoma. J Immunother Cancer. 2022;10:e004026.
Ri MH, Ma J, Jin X. Development of natural products for anti-PD-1/PD-L1 immunotherapy against cancer. J Ethnopharmacol. 2021;281:114370.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28.
Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529.
Yi M, Niu M, Xu L, Luo S, Wu K. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14:10.
Monaco L, De Bernardi E, Bono F. The, “digital biopsy” in non-small cell lung cancer (NSCLC): a pilot study to predict the PD-L1 status from radiomics features of [18F]FDG PET/CT. Eur J Nucl Med Mol Imaging. 2022;49:3401–11.
Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol. 2020;15:499–519.
Nimmagadda S. Quantifying PD-L1 expression to monitor immune checkpoint therapy: opportunities and challenges. Cancers. 2020;12:3173.
Yeong J, Lum HYJ, Teo CB, Tan BKJ, Chan YH, Tay RYK, et al. Choice of PD-L1 immunohistochemistry assay influences clinical eligibility for gastric cancer immunotherapy. Gastric Cancer. 2022;25:741–50.
Yoon JY, Nayyar R, Quest G, Pabedinskas D, Pal P, Tsao MS, et al. PD-L1 lineage-specific quantification in malignant pleural effusions of lung adenocarcinoma by flow cytometry. Lung Cancer. 2020;148:55–61.
Christensen C, Kristensen LK, Alfsen MZ, Nielsen CH, Kjaer A. Quantitative PET imaging of PD-L1 expression in xenograft and syngeneic tumour models using a site-specifically labelled PD-L1 antibody. Eur J Nucl Med Mol Imaging. 2020;47:1302–13.
Zhou H, Bao G, Wang Z, Zhang B, Li D, Chen L, et al. PET imaging of an optimized anti-PD-L1 probe 68Ga-NODAGA-BMS986192 in immunocompetent mice and non-human primates. EJNMMI Res. 2022;12:35.
Zhou M, Wang X, Chen B, Xiang S, Rao W, Zhang Z, et al. Preclinical and first-in-human evaluation of 18F-labeled D-peptide antagonist for PD-L1 status imaging with PET. Eur J Nucl Med Mol Imaging. 2022;49:4312–24.
Li D, Wang F, Jiang J, Hou X, Ding J, Wang Z, et al. Construction of an iodine-labeled CS1001 antibody for targeting PD-L1 detection and comparison with low-molecular-peptide micro-PET imaging. Mol Pharm. 2022;19:4382–9.
Lv G, Sun X, Qiu L, Sun Y, Li K, Liu Q, et al. PET imaging of tumor PD-L1 expression with a highly specific nonblocking single-domain antibody. J Nucl Med. 2020;61:117–22.
Chen Y, Zhu S, Fu J, Lin J, Sun Y, Lv G, et al. Development of a radiolabeled site-specific single-domain antibody positron emission tomography probe for monitoring PD-L1 expression in cancer. J Pharm Anal. 2022;12:869–78.
Liu Q, Jiang L, Li K, Li H, Lv G, Lin J, et al. Immuno-PET imaging of 68Ga-labeled nanobody Nb109 for dynamic monitoring the PD-L1 expression in cancers. Cancer Immunol Immunoth. 2021;70:1721–33.
Wen X, Zeng X, Cheng X, Zeng X, Liu J, Zhang Y, et al. PD-L1-targeted radionuclide therapy combined with αPD-L1 antibody immunotherapy synergistically improves the antitumor effect. Mol Pharm. 2022;19:3612–22.
Cheng Y, Shi D, Xu Z, Gao Z, Si Z, Zhao Y, et al. 124I-labeled monoclonal antibody and fragment for the noninvasive evaluation of tumor PD-L1 expression in vivo. Mol Pharm. 2022;19:3551–62.
Moroz A, Lee CY, Wang YH, Hsiao JC, Sevillano N, Truillet C, et al. A preclinical assessment of 89Zr-atezolizumab identifies a requirement for carrier added formulations not observed with 89Zr-C4. Bioconjug Chem. 2018;29:3476–82.
Jung KH, Park JW, Lee JH, Moon SH, Cho YS, Lee KH. 89Zr-labeled anti-PD-L1 antibody PET monitors gemcitabine therapy-induced modulation of tumor PD-L1 expression. J Nucl Med. 2021;62:656–64.
Cheng Y, Shi D, Ye R, Fu W, Ma P, Si Z, et al. Noninvasive evaluation of PD-L1 expression in non-small cell lung cancer by immunoPET imaging using an acylating agent-modified antibody fragment. Eur J Nucl Med Mol Imaging. 2023;50:1585–96.
Hu K, Kuan H, Hanyu M, Masayuki H, Xie L, Zhang Y, et al. Developing native peptide-based radiotracers for PD-L1 PET imaging and improving imaging contrast by pegylation. Chem Commun. 2019;55:4162–5.
De Silva RA, Kumar D, Lisok A, Chatterjee S, Wharram B, Venkateswara Rao K, et al. Peptide-based 68Ga-PET radiotracer for imaging PD-L1 expression in cancer. Mol Pharm. 2018;15:3946–52.
Zhou X, Jiang J, Yang X, Liu T, Ding J, Nimmagadda S, et al. First-in-humans evaluation of a PD-L1-binding peptide PET radiotracer in non-small cell lung cancer patients. J Nucl Med. 2022;63:536–42.
Kumar D, Mishra A, Lisok A, Kureshi R, Shelake S, Plyku D, et al. Pharmacodynamic measures within tumors expose differential activity of PD(L)-1 antibody therapeutics. Proc Natl Acad Sci U S A. 2021;118:e2107982118.
Zou S, Liu J, Sun Z, Feng X, Wang Z, Jin Y, et al. Discovery of hPRDX5-based peptide inhibitors blocking PD-1/PD-L1 interaction through in silico proteolysis and rational design. Cancer Chemotherapy Pharmacol. 2020;85:185–93.
Pauwels E, Cleeren F, Tshibangu T, Koole M, Serdons K, Boeckxstaens L, et al. 18F-AlF-NOTA-octreotide outperforms 68Ga-DOTATATE/NOC PET in neuroendocrine tumor patients: results from a prospective. Multicenter Study J Nucl Med. 2023;64:632–8.
Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022;19:287–305.
Luo H, Yang C, Kuang D, Shi S, Chan AW. Visualizing dynamic changes in PD-L1 expression in non-small cell lung carcinoma with radiolabeled recombinant human PD-1. Eur J Nucl Med Mol Imaging. 2022;49:2735–45.
Chen J, Chen R, Huang S, Zu B, Zhang S. Atezolizumab alleviates the immunosuppression induced by PD-L1-positive neutrophils and improves the survival of mice during sepsis. Mol Med Rep. 2021;23:144.
Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–39.
Deberle L, Benešová M, Umbricht C, Borgna F, Büchler M, Zhernosekov K, et al. Development of a new class of PSMA radioligands comprising ibuprofen as an albumin-binding entity. Theranostics. 2020;10(4):1678–93.
Funding
This study was financially supported by National Natural Science Foundation of China (22076069), Natural Science Foundation of Jiangsu Province (BK20201135), the Major Scientific Research Project of Jiangsu Commission of Health (ZDA2020007, ZD2022036) and the Science Technology and Development Project of Wuxi (Y20212013). We also acknowledge the financial support received from Jiangsu Provincial Medical Key Laboratory (Key Laboratory of Nuclear Medicine).
Author information
Authors and Affiliations
Contributions
SZ: experiments, data analysis, manuscript writing; BL: experiments, data analysis and manuscript writing; YZ: experiments and data analysis; YC: experiments and data analysis; JF: experiments and data analysis; LQ and JL: design, supervision, manuscript writing and editing. All authors reviewed and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval
All procedures involving animal studies were reviewed and approved by the Animal Care and Ethics Committee of Jiangsu Institute of Nuclear Medicine (JSINM-2022–014).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, S., Liang, B., Zhou, Y. et al. Development of novel peptide-based radiotracers for detecting PD-L1 expression and guiding cancer immunotherapy. Eur J Nucl Med Mol Imaging 51, 625–640 (2024). https://doi.org/10.1007/s00259-023-06480-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06480-1