Abstract
Purpose
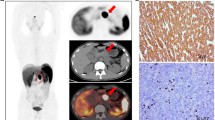
To explore the role of fully hybrid 68Ga-DOTATOC PET/MR imaging and radiomic parameters in predicting histopathological prognostic factors in patients with pancreatic neuroendocrine tumours (PanNETs) undergoing surgery.
Methods
One hundred eighty-seven consecutive 68Ga-DOTATOC PET/MRI scans (March 2018–June 2020) performed for gastroenteropancreatic neuroendocrine tumour were retrospectively evaluated; 16/187 patients met the eligibility criteria (68Ga-DOTATOC PET/MRI for preoperative staging of PanNET and availability of histological data). PET/MR scans were qualitatively and quantitatively interpreted, and the following imaging parameters were derived: PET-derived SUVmax, SUVmean, somatostatin receptor density (SRD), total lesion somatostatin receptor density (TLSRD), and MRI-derived apparent diffusion coefficient (ADC), arterial and late enhancement, necrosis, cystic degeneration, and maximum diameter. Additionally, first-, second-, and higher-order radiomic parameters were extracted from both PET and MRI scans. Correlations with several PanNETs’ histopathological prognostic factors were evaluated using Spearman’s coefficient, while the area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to evaluate parameters’ predictive performance.
Results
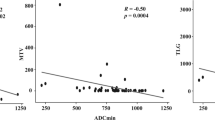
Primary tumour was detected in all 16 patients (15/16 by 68Ga-DOTATOC PET and 16/16 by MRI). SUVmax and SUVmean resulted good predictors of lymphnodal (LN) involvement (AUC of 0.850 and 0.783, respectively). Second-order radiomic parameters GrayLevelVariance and HighGrayLevelZoneEmphasis extracted from T2 MRI demonstrated significant correlations with LN involvement (adjusted p = 0.009), also showing good predictive performance (AUC = 0.992).
Conclusion
This study demonstrates the role of the fully hybrid PET/MRI tool for the synergic function of imaging parameters extracted by the two modalities and highlights the potentiality of imaging and radiomic parameters in assessing histopathological features of PanNET aggressiveness.






Similar content being viewed by others
References
Klimstra DS, Beltran H, Lilenbaum R, Bergsland E. The spectrum of neuroendocrine tumors: histologic classification, unique features and areas of overlap. Am Soc Clin Oncol Educ Book. 2015:92–103. https://doi.org/10.14694/EdBook_AM.2015.35.92.
Sun J. Pancreatic neuroendocrine tumors. Intractable Rare Dis Res. 2017;6:21–8. https://doi.org/10.5582/irdr.2017.01007.
Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with (68)Ga-DOTA-conjugated somatostatin receptor targeting peptides and (18)F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44:1588–601. https://doi.org/10.1007/s00259-017-3728-y.
Ambrosini V, Campana D, Polverari G, Peterle C, Diodato S, Ricci C, et al. Prognostic value of 68Ga-DOTANOC PET/CT SUVmax in patients with neuroendocrine tumors of the pancreas. J Nucl Med. 2015;56:1843–8. https://doi.org/10.2967/jnumed.115.162719.
Mapelli P, Partelli S, Salgarello M, Doraku J, Muffatti F, Schiavo Lena M, et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET improve preoperative evaluation of aggressiveness in resectable pancreatic neuroendocrine neoplasms. Diagnostics (Basel). 2021;11. https://doi.org/10.3390/diagnostics11020192.
Mapelli P, Partelli S, Salgarello M, Doraku J, Pasetto S, Rancoita PMV, et al. Dual tracer 68Ga-DOTATOC and 18F-FDG PET/computed tomography radiomics in pancreatic neuroendocrine neoplasms: an endearing tool for preoperative risk assessment. Nucl Med Commun. 2020;41:896–905. https://doi.org/10.1097/MNM.0000000000001236.
Pichler BJ, Wehrl HF, Kolb A, Judenhofer MS. Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging? Semin Nucl Med. 2008;38:199–208. https://doi.org/10.1053/j.semnuclmed.2008.02.001.
Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. Radiographics. 2015;35:500–16. https://doi.org/10.1148/rg.352140164.
Hope TA, Pampaloni MH, Nakakura E, VanBrocklin H, Slater J, Jivan S, et al. Simultaneous (68)Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom Imaging. 2015;40:1432–40. https://doi.org/10.1007/s00261-015-0409-9.
Mapelli P, De Cobelli, F, Picchio M. PET/MRI in neuroendocrine tumours: blessings and curses. Current Radiopharmaceuticals. 2019;12.
Mapelli P, Ironi G, Fallanca F, Partelli S, Muffatti F, Andreasi V, Gianolli L, Falconi M, De Cobelli F, Picchio M. 68Ga-DOTA-peptides PET/MRI in pancreatico-duodenal neuroendocrine tumours: a flash pictorial essay on assets and lacks. Clin Transl Imaging. 2019;7:363–71.
Humphrey PE, Alessandrino F, Bellizzi AM, Mortele KJ. Non-hyperfunctioning pancreatic endocrine tumors: multimodality imaging features with histopathological correlation. Abdom Imaging. 2015;40:2398–410. https://doi.org/10.1007/s00261-015-0458-0.
Oksuz MO, Winter L, Pfannenberg C, Reischl G, Mussig K, Bares R, et al. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn Interv Imaging. 2014;95:289–300. https://doi.org/10.1016/j.diii.2013.07.006.
Rha SE, Jung SE, Lee KH, Ku YM, Byun JY, Lee JM. CT and MR imaging findings of endocrine tumor of the pancreas according to WHO classification. Eur J Radiol. 2007;62:371–7. https://doi.org/10.1016/j.ejrad.2007.02.036.
Mayerhoefer ME, Materka A, Langs G, Haggstrom I, Szczypinski P, Gibbs P, et al. Introduction to radiomics. J Nucl Med. 2020;61:488–95. https://doi.org/10.2967/jnumed.118.222893.
Bezzi C, Mapelli P, Presotto L, Neri I, Scifo P, Savi A, et al. Radiomics in pancreatic neuroendocrine tumors: methodological issues and clinical significance. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05338-8
Bevilacqua A, Calabro D, Malavasi S, Ricci C, Casadei R, Campana D, et al. A [68Ga]Ga-DOTANOC PET/CT radiomic model for non-invasive prediction of tumour grade in pancreatic neuroendocrine tumours. Diagnostics (Basel). 2021;11. https://doi.org/10.3390/diagnostics11050870.
Onner H, Abdulrezzak U, Tutus A. Could the skewness and kurtosis texture parameters of lesions obtained from pretreatment Ga-68 DOTA-TATE PET/CT images predict receptor radionuclide therapy response in patients with gastroenteropancreatic neuroendocrine tumors? Nucl Med Commun. 2020;41:1034–9. https://doi.org/10.1097/MNM.0000000000001231.
Weber M, Kessler L, Schaarschmidt B, Fendler WP, Lahner H, Antoch G, et al. Treatment-related changes in neuroendocrine tumors as assessed by textural features derived from (68)Ga-DOTATOC PET/MRI with simultaneous acquisition of apparent diffusion coefficient. BMC Cancer. 2020;20:326. https://doi.org/10.1186/s12885-020-06836-y.
Werner RA, Ilhan H, Lehner S, Papp L, Zsoter N, Schatka I, et al. Pre-therapy somatostatin receptor-based heterogeneity predicts overall survival in pancreatic neuroendocrine tumor patients undergoing peptide receptor radionuclide therapy. Mol Imaging Biol. 2019;21:582–90. https://doi.org/10.1007/s11307-018-1252-5.
Bruckmann NM, Rischpler C, Kirchner J, Umutlu L, Herrmann K, Ingenwerth M, et al. Correlation between contrast enhancement, standardized uptake value (SUV), and diffusion restriction (ADC) with tumor grading in patients with therapy-naive neuroendocrine neoplasms using hybrid (68)Ga-DOTATOC PET/MRI. Eur J Radiol. 2021;137:109588. https://doi.org/10.1016/j.ejrad.2021.109588.
Taha AA, Hanbury A. Metrics for evaluating 3D medical image segmentation: analysis, selection, and tool. BMC Med Imaging. 2015;15:29. https://doi.org/10.1186/s12880-015-0068-x.
Klimstra DS, Kloppell G, La Rosa S and Rindi G. Digestive system tumours. WHO classification of tumours 2019;1. IARC, Lyon.
Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. https://doi.org/10.1016/j.jcm.2016.02.012.
Curran T, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N. Importance of lymph node involvement in pancreatic neuroendocrine tumors: impact on survival and implications for surgical resection. J Gastrointest Surg. 2015;19:152–60; discussion 60. https://doi.org/10.1007/s11605-014-2624-z.
Ellison TA, Wolfgang CL, Shi C, Cameron JL, Murakami P, Mun LJ, et al. A single institution’s 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg. 2014;259:204–12. https://doi.org/10.1097/SLA.0b013e31828f3174.
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–71. https://doi.org/10.1159/000443171.
Partelli S, Javed AA, Andreasi V, He J, Muffatti F, Weiss MJ, et al. The number of positive nodes accurately predicts recurrence after pancreaticoduodenectomy for nonfunctioning neuroendocrine neoplasms. Eur J Surg Oncol. 2018;44:778–83. https://doi.org/10.1016/j.ejso.2018.03.005.
Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–55. https://doi.org/10.1002/cncr.23469.
Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017;15:133. https://doi.org/10.1186/s12916-017-0900-y.
Stanta G, Bonin S. Overview on clinical relevance of intra-tumor heterogeneity. Front Med (Lausanne). 2018;5:85. https://doi.org/10.3389/fmed.2018.00085.
Zwanenburg A, Vallieres M, Abdalah MA, Aerts H, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38. https://doi.org/10.1148/radiol.2020191145.
Noble WS. How does multiple testing correction work? Nat Biotechnol. 2009;27:1135–7. https://doi.org/10.1038/nbt1209-1135.
De Robertis R, Maris B, Cardobi N, Tinazzi Martini P, Gobbo S, Capelli P, et al. Can histogram analysis of MR images predict aggressiveness in pancreatic neuroendocrine tumors? Eur Radiol. 2018;28:2582–91. https://doi.org/10.1007/s00330-017-5236-7.
Bian Y, Zhao Z, Jiang H, Fang X, Li J, Cao K, et al. Noncontrast radiomics approach for predicting grades of nonfunctional pancreatic neuroendocrine tumors. J Magn Reson Imaging. 2020;52:1124–36. https://doi.org/10.1002/jmri.27176.
Abdulrezzak U, Kurt YK, Kula M, Tutus A. Combined imaging with 68Ga-DOTA-TATE and 18F-FDG PET/CT on the basis of volumetric parameters in neuroendocrine tumors. Nucl Med Commun. 2016;37:874–81. https://doi.org/10.1097/MNM.0000000000000522.
Campana D, Ambrosini V, Pezzilli R, Fanti S, Labate AM, Santini D, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med. 2010;51:353–9. https://doi.org/10.2967/jnumed.109.066662.
Acknowledgements
The SIGNA PET/MRI system (General Electric Healthcare, Waukesha, WI, USA) used in the present work has been purchased with funding from the Italian Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
All patients gave their informed consent to participate to the study.
Conflict of interest
Prof. Massimo Falconi is Advisory Board Member of Advanced Accelerator Application (AAA). All the other authors have no conflicts of interest related to the present paper to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - General.
Rights and permissions
About this article
Cite this article
Mapelli, P., Bezzi, C., Palumbo, D. et al. 68Ga-DOTATOC PET/MR imaging and radiomic parameters in predicting histopathological prognostic factors in patients with pancreatic neuroendocrine well-differentiated tumours. Eur J Nucl Med Mol Imaging 49, 2352–2363 (2022). https://doi.org/10.1007/s00259-022-05677-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05677-0