Abstract
Purpose
To investigate the performance of short-time dynamic imaging in quantifying kinetic metrics of 2-[18F]-fluoro-2-deoxy-d-glucose (18F-FDG).
Methods
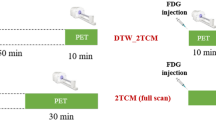
Dynamic total-body positron emission tomography (PET) imaging was performed in 11 healthy volunteers for 75 min. The data were divided into 30-, 45- and 75-min groups. Nonlinear regression (NLR) generated constant rates (k1 to k3) and NLR-based Ki in various organs. The Patlak method calculated parametric Ki images to generate Patlak-based Ki values. Paired samples t-test or the Wilcoxon signed-rank test compared the kinetic metrics between the groups, depending on data normality. Deming regression and Bland–Altman analysis assessed the correlation and agreement between NLR- and Patlak-based Ki. A two-sided P < 0.05 was considered statistically significant.
Results
The 45- and 75-min groups were similar in NLR-based kinetic metrics. The relative difference ranges were as follows: k1, from 3.4% (P = 0.627) in the spleen to 57.9% (P = 0.130) in the white matter; k2, from 6.0% (P = 0.904) in the spleen to 60.7% (P = 0.235) in the left ventricle (LV) myocardium; k3, from 45.6% (P = 0.302) in the LV myocardium to 96.3% (P = 0.478) in the liver; Ki, from 14.0% (P = 0.488) in the liver to 77.8% (P = 0.067) in the kidney. Patlak-based Ki values were also similar between these groups in all organs, except the grey matter (9.6%, P = 0.029) and cerebellum (14.4%, P = 0.002). However, significant differences in kinetic metrics were found between the 30-min and 75-min groups in most organs both in NLR- and Patlak-based analyses. The NLR- and Patlak-based Ki values significantly correlated, with no bias in any of the organs.
Conclusion
Dynamic imaging using a high-sensitivity total-body PET scanner for a shorter time of 45 min could achieve relevant kinetic metrics of 18F-FDG as done by long-time imaging.






Similar content being viewed by others
Availability of data and material
The dataset used and/or analysed during the current study is available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci USA. 2000;97(16):9226–33.
Zhang X, Xie Z, Berg E, Judenhofer MS, Liu W, Xu T, et al. Total-body dynamic reconstruction and parametric imaging on the uEXPLORER. J Nucl Med. 2020;61(2):285–91.
Dunnwald LK, Doot RK, Specht JM, Gralow JR, Ellis GK, Livingston RB, et al. PET tumor metabolism in locally advanced breast cancer patients undergoing neoadjuvant chemotherapy: value of static versus kinetic measures of fluorodeoxyglucose uptake. Clin Cancer Res. 2011;17(8):2400–9.
Karakatsanis NA, Lodge MA, Tahari AK, Zhou Y, Wahl RL, Rahmim A. Dynamic whole-body PET parametric imaging: I. concept, acquisition protocol optimization and clinical application. Phys Med Biol. 2013;58(20):7391–418.
Strauss LG, Klippel S, Pan L, Schonleben K, Haberkorn U, Dimitrakopoulou-Strauss A. Assessment of quantitative FDG PET data in primary colorectal tumours: which parameters are important with respect to tumour detection? Eur J Nucl Med Mol Imaging. 2007;34(6):868–77.
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H, Burger C, Bernd L, et al. The role of quantitative (18)F-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med. 2002;43(4):510–8.
Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M, Burger C, Heichel T, Willeke F, et al. Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J Nucl Med. 2001;42(5):713–20.
Rusten E, Rodal J, Revheim ME, Skretting A, Bruland OS, Malinen E. Quantitative dynamic (1)(8)FDG-PET and tracer kinetic analysis of soft tissue sarcomas. Acta Oncol. 2013;52(6):1160–7.
Strauss LG, Dimitrakopoulou-Strauss A, Koczan D, Bernd L, Haberkorn U, Ewerbeck V, et al. 18F-FDG kinetics and gene expression in giant cell tumors. J Nucl Med. 2004;45(9):1528–35.
Dimitrakopoulou-Strauss A, Hohenberger P, Pan L, Kasper B, Roumia S, Strauss LG. Dynamic PET with FDG in patients with unresectable aggressive fibromatosis: regression-based parametric images and correlation to the FDG kinetics based on a 2-tissue compartment model. Clin Nucl Med. 2012;37(10):943–8.
Cochet A, Pigeonnat S, Khoury B, Vrigneaud JM, Touzery C, Berriolo-Riedinger A, et al. Evaluation of breast tumor blood flow with dynamic first-pass 18F-FDG PET/CT: comparison with angiogenesis markers and prognostic factors. J Nucl Med. 2012;53(4):512–20.
Dimitrakopoulou-Strauss A, Strauss LG, Burger C, Ruhl A, Irngartinger G, Stremmel W, et al. Prognostic aspects of 18F-FDG PET kinetics in patients with metastatic colorectal carcinoma receiving FOLFOX chemotherapy. J Nucl Med. 2004;45(9):1480–7.
Dimitrakopoulou-Strauss A, Strauss LG, Egerer G, Vasamiliette J, Mechtersheimer G, Schmitt T, et al. Impact of dynamic 18F-FDG PET on the early prediction of therapy outcome in patients with high-risk soft-tissue sarcomas after neoadjuvant chemotherapy: a feasibility study. J Nucl Med. 2010;51(4):551–8.
Dimitrakopoulou-Strauss A, Strauss LG, Egerer G, Vasamiliette J, Schmitt T, Haberkorn U, et al. Prediction of chemotherapy outcome in patients with metastatic soft tissue sarcomas based on dynamic FDG PET (dPET) and a multiparameter analysis. Eur J Nucl Med Mol Imaging. 2010;37(8):1481–9.
Humbert O, Lasserre M, Bertaut A, Fumoleau P, Coutant C, Brunotte F, et al. Breast cancer blood flow and metabolism on dual-acquisition (18)F-FDG PET: correlation with tumor phenotype and neoadjuvant chemotherapy response. J Nucl Med. 2018;59(7):1035–41.
Ilan E, Sandstrom M, Velikyan I, Sundin A, Eriksson B, Lubberink M. Parametric net influx rate images of (68)Ga-DOTATOC and (68)Ga-DOTATATE: quantitative accuracy and improved image contrast. J Nucl Med. 2017;58(5):744–9.
Karakatsanis NA, Zhou Y, Lodge MA, Casey ME, Wahl RL, Zaidi H, et al. Generalized whole-body Patlak parametric imaging for enhanced quantification in clinical PET. Phys Med Biol. 2015;60(22):8643–73.
Dimitrakopoulou-Strauss A, Pan L, Sachpekidis C. Kinetic modeling and parametric imaging with dynamic PET for oncological applications: general considerations, current clinical applications, and future perspectives. Eur J Nucl Med Mol Imaging. 2021;48(1):21–39.
Rahmim A, Lodge MA, Karakatsanis NA, Panin VY, Zhou Y, McMillan A, et al. Dynamic whole-body PET imaging: principles, potentials and applications. Eur J Nucl Med Mol Imaging. 2019;46(2):501–18.
Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, et al. First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med. 2019;60(3):299–303.
Kimura N, Yamamoto Y, Kameyama R, Hatakeyama T, Kawai N, Nishiyama Y. Diagnostic value of kinetic analysis using dynamic 18F-FDG-PET in patients with malignant primary brain tumor. Nucl Med Commun. 2009;30(8):602–9.
Wu H, Dimitrakopoulou-Strauss A, Heichel TO, Lehner B, Bernd L, Ewerbeck V, et al. Quantitative evaluation of skeletal tumours with dynamic FDG PET: SUV in comparison to Patlak analysis. Eur J Nucl Med. 2001;28(6):704–10.
Mizrahi R, Rusjan PM, Vitcu I, Ng A, Wilson AA, Houle S, et al. Whole body biodistribution and radiation dosimetry in humans of a new PET ligand, [(18)F]-FEPPA, to image translocator protein (18 kDa). Mol Imaging Biol. 2013;15(3):353–9.
Fujimura Y, Kimura Y, Simeon FG, Dickstein LP, Pike VW, Innis RB, et al. Biodistribution and radiation dosimetry in humans of a new PET ligand, 18F-PBR06, to image translocator protein (18 kDa). J Nucl Med. 2010;51(1):145–9.
Zhu W, Li Q, Bai B, Conti PS, Leahy RM. Patlak image estimation from dual time-point list-mode PET data. IEEE Trans Med Imaging. 2014;33(4):913–24.
Karakatsanis NA, Casey ME, Lodge MA, Rahmim A, Zaidi H. Whole-body direct 4D parametric PET imaging employing nested generalized Patlak expectation-maximization reconstruction. Phys Med Biol. 2016;61(15):5456–85.
Strauss LG, Pan L, Cheng C, Haberkorn U, Dimitrakopoulou-Strauss A. Shortened acquisition protocols for the quantitative assessment of the 2-tissue-compartment model using dynamic PET/CT 18F-FDG studies. J Nucl Med. 2011;52(3):379–85.
Cherry SR, Badawi RD, Karp JS, Moses WW, Price P, Jones T. Total-body imaging: transforming the role of positron emission tomography. Sci Transl Med. 2017;9(381):eaaf6169.
Zhang X, Badawi RD, Cherry SR, Qi J. Theoretical study of the benefit of long axial field-of-view PET on region of interest quantification. Phys Med Biol. 2018;63(13):767–70.
Zhang Y, Hu P, Wu R, Gu Y, Chen S, Yu H, et al. The image quality, lesion detectability, and acquisition time of 18F-FDG total-body PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2020;47(11):2507–15.
Zhang X, Zhou J, Cherry SR, Badawi RD, Qi J. Quantitative image reconstruction for total-body PET imaging using the 2-meter long EXPLORER scanner. Phys Med Biol. 2017;62(6):2465–85.
Liu G, Xu H, Hu P, Tan H, Zhang Y, Yu H, et al. Kinetic metrics of (18)F-FDG in normal human organs identified by systematic dynamic total-body positron emission tomography. Eur J Nucl Med Mol Imaging. 2021;48(8):2363–72.
Liu G, Hu P, Yu H, Tan H, Zhang Y, Yin H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48(8):2373–83.
Wahl LM, Asselin MC, Nahmias C. Regions of interest in the venous sinuses as input functions for quantitative PET. J Nucl Med. 1999;40(10):1666–75.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations J Cereb Blood Flow Metab. 1985;5(4):584–90.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Feng T, Zhao Y, Shi H, Li H, Zhang X, Wang G, et al. Total-body quantitative parametric imaging of early kinetics of FDG. J Nucl Med. 2021;62(5):738–44.
Pan L, Cheng C, Haberkorn U, Dimitrakopoulou-Strauss A. Machine learning-based kinetic modeling: a robust and reproducible solution for quantitative analysis of dynamic PET data. Phys Med Biol. 2017;62(9):3566–81.
Zuo Y, Sarkar S, Corwin MT, Olson K, Badawi RD, Wang G. Structural and practical identifiability of dual-input kinetic modeling in dynamic PET of liver inflammation. Phys Med Biol. 2019;64(17):175023.
Brix G, Ziegler SI, Bellemann ME, Doll J, Schosser R, Lucht R, et al. Quantification of [(18)F]FDG uptake in the normal liver using dynamic PET: impact and modeling of the dual hepatic blood supply. J Nucl Med. 2001;42(8):1265–73.
de Geus-Oei LF, Visser EP, Krabbe PF, van Hoorn BA, Koenders EB, Willemsen AT, et al. Comparison of image-derived and arterial input functions for estimating the rate of glucose metabolism in therapy-monitoring 18F-FDG PET studies. J Nucl Med. 2006;47(6):945–9.
Funding
This study was supported by grants from the Shanghai “Rising Stars of Medical Talent”–Youth Development Program (HWJRS2019-72), the Training Program for Excellent Young Medical Talents of Zhongshan Hospital of Fudan University (2019ZSYQ28), the Shanghai Municipal Key Clinical Specialty Project (SHSLCZDZK03401), the Major Science and Technology Projects for Major New Drug Creation (2019ZX09302001), the Shanghai Science and Technology Committee Program (20DZ2201800), the Three-year Action Plan of Clinical Skills and Innovation of Shanghai Hospital Development Center (SHDC2020CR3079B), and the Next Generation Information Infrastructure Construction Project founded by Shanghai Municipal Commission of Economy and Informatization (201901014).
Author information
Authors and Affiliations
Contributions
G. Liu and H. Yu had full access to all the study data and take full responsibility for its integrity. G. Liu and H. Shi conceptualised and designed the study. G. Liu, P. Hu, D. Shi, H. Yin, and H. Tan acquired the data. G. Liu, P. Hu, Y. Hu, and Y. Zhang analysed and interpreted the data. G. Liu and H. Shi drafted the manuscript. All authors critically revised the manuscript. G. Liu, D. Shi, and Y. Hu did the statistical analysis. G. Liu, Y. Zhang, and H. Shi secured funding.
Corresponding author
Ethics declarations
Ethics approval
The Ethics Committee of Zhongshan Hospital of Fudan University approved this study (approval number: IRB2015-098).
Consent to participate
Written informed consent was obtained from included subjects for participation of this study.
Consent for publication
The authors affirm that the human research participants provided informed consent for publication of the studied data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Miscellanea
Rights and permissions
About this article
Cite this article
Liu, G., Yu, H., Shi, D. et al. Short-time total-body dynamic PET imaging performance in quantifying the kinetic metrics of 18F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging 49, 2493–2503 (2022). https://doi.org/10.1007/s00259-021-05500-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05500-2