Abstract
Purpose
We evaluated whether biomarkers on baseline [18F]-FDG PET/CT are associated with recurrence after surgery in patients with invasive breast cancer of no special type (NST).
Methods
In this retrospective single-center study, we included consecutive patients with non-metastatic breast cancer of NST who underwent [18F]-FDG PET/CT before treatment, including surgery, between 2011 and 2016. Clinicopathological data were collected. Tumor SUVmax, total metabolic tumor volume (TMTV), and spleen- and bone marrow-to-liver SUVmax ratios (SLR, BLR) were measured from the PET images. Cut-off values were determined using predictiveness curves to predict 5-year recurrence-free survival (5y-RFS). A multivariable prediction model was developed using Cox regression. The association with stromal tumor-infiltrating lymphocytes (TILs) levels (low if <50%) was studied by logistic regression.
Results
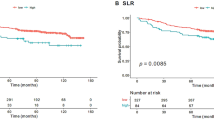
Three hundred and three women were eligible, including 93 (31%) with triple-negative breast carcinoma. After a median follow-up of 6.2 years, 56 and 35 patients experienced recurrence and death, respectively. The 5y-RFS rate was 86%. In multivariable analyses, high TMTV (>20 cm3) and high SLR (>0.76) were associated with shorter 5y-RFS (HR 2.4, 95%CI 1.3–4.5, and HR 1.9, 95%CI 1.0–3.6). In logistic regression, high SLR was the only independent factor associated with low stromal TILs (OR 2.8, 95%CI 1.4–5.7).
Conclusion
High total metabolic tumor volume and high spleen glucose metabolism on baseline [18F]-FDG PET/CT were associated with poor 5y-RFS after surgical resection in patients with breast cancer of NST. Spleen metabolism was inversely correlated with stromal TILs and might be a surrogate for an immunosuppressive tumor microenvironment.




Similar content being viewed by others
References
Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res. 2007;13:3207–14.
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh I-T, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
Lee SH, Ha S, An HJ, Lee JS, Han W, Im S-A, et al. Association between partial-volume corrected SUVmax and Oncotype DX recurrence score in early-stage, ER-positive/HER2-negative invasive breast cancer. Eur J Nucl Med Mol Imaging. 2016;43:1574–84.
Buus R, Sestak I, Kronenwett R, Ferree S, Schnabel CA, Baehner FL, et al. Molecular drivers of Oncotype DX, Prosigna, EndoPredict, and the Breast Cancer Index: a TransATAC study. J Clin Oncol. 2021;39:126–35.
Ko H, Baghdadi Y, Love C, Sparano JA. Clinical utility of 18F-FDG PET/CT in staging localized breast cancer before initiating preoperative systemic therapy. J Natl Compr Cancer Netw. 2020;18:1240–6.
Hyland CJ, Varghese F, Yau C, Beckwith H, Khoury K, Varnado W, et al. Use of 18F-FDG PET/CT as an initial staging procedure for stage II-III breast cancer: a multicenter value analysis. J Natl Compr Cancer Netw. 2020;18:1510–7.
Nakajo M, Kajiya Y, Kaneko T, Kaneko Y, Takasaki T, Tani A, et al. FDG PET/CT and diffusion-weighted imaging for breast cancer: prognostic value of maximum standardized uptake values and apparent diffusion coefficient values of the primary lesion. Eur J Nucl Med Mol Imaging. 2010;37:2011–20.
Groheux D, Giacchetti S, Moretti J-L, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Koo HR, Park JS, Kang KW, Han W, Park IA, Moon WK. Correlation between (18)F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur Radiol. 2015;25:3314–21.
Nishimukai A, Inoue N, Kira A, Takeda M, Morimoto K, Araki K, et al. Tumor size and proliferative marker geminin rather than Ki67 expression levels significantly associated with maximum uptake of 18F-deoxyglucose levels on positron emission tomography for breast cancers. PLoS One. 2017;12:e0184508.
Kitajima K, Yamano T, Fukushima K, Miyoshi Y, Hirota S, Kawanaka Y, et al. Correlation of the SUVmax of FDG-PET and ADC values of diffusion-weighted MR imaging with pathologic prognostic factors in breast carcinoma. Eur J Radiol. 2016;85:943–9.
Yoo J, Yoon H-J, Kim BS. Prognostic value of primary tumor SUVmax on F-18 FDG PET/CT compared with semi-quantitative tumor uptake on Tc-99m sestamibi breast-specific gamma imaging in invasive ductal breast cancer. Ann Nucl Med. 2017;31:19–28.
Diao W, Tian F, Jia Z. The prognostic value of SUVmax measuring on primary lesion and ALN by 18F-FDG PET or PET/CT in patients with breast cancer. Eur J Radiol. 2018;105:1–7.
Jiménez-Ballvé A, García García-Esquinas M, Salsidua-Arroyo O, Serrano-Palacio A, García-Sáenz JA, Ortega Candil A, et al. Prognostic value of metabolic tumour volume and total lesion glycolysis in 18F-FDG PET/CT scans in locally advanced breast cancer staging. Rev Esp Med Nucl Imagen Mol. 2016;35:365–72.
Higuchi T, Fujimoto Y, Ozawa H, Bun A, Fukui R, Miyagawa Y, et al. Significance of metabolic tumor volume at baseline and reduction of mean standardized uptake value in 18F-FDG-PET/CT imaging for predicting pathological complete response in breast cancers treated with preoperative chemotherapy. Ann Surg Oncol. 2019;26:2175–83.
Lemarignier C, Martineau A, Teixeira L, Vercellino L, Espié M, Merlet P, et al. Correlation between tumour characteristics, SUV measurements, metabolic tumour volume, TLG and textural features assessed with 18F-FDG PET in a large cohort of oestrogen receptor-positive breast cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:1145–54.
Seban R-D, Robert C, Dercle L, Yeh R, Dunant A, Reuze S, et al. Increased bone marrow SUVmax on 18F-FDG PET is associated with higher pelvic treatment failure in patients with cervical cancer treated by chemoradiotherapy and brachytherapy. Oncoimmunology. 2019;8:e1574197.
Seban R-D, Nemer JS, Marabelle A, Yeh R, Deutsch E, Ammari S, et al. Prognostic and theranostic 18F-FDG PET biomarkers for anti-PD1 immunotherapy in metastatic melanoma: association with outcome and transcriptomics. Eur J Nucl Med Mol Imaging. 2019;46:2298–310.
Seban R-D, Moya-Plana A, Antonios L, Yeh R, Marabelle A, Deutsch E, et al. Prognostic 18F-FDG PET biomarkers in metastatic mucosal and cutaneous melanoma treated with immune checkpoint inhibitors targeting PD-1 and CTLA-4. Eur J Nucl Med Mol Imaging. 2020.
Wong A, Callahan J, Keyaerts M, Neyns B, Mangana J, Aberle S, et al. 18F-FDG PET/CT based spleen to liver ratio associates with clinical outcome to ipilimumab in patients with metastatic melanoma. Cancer Imaging. 2020;20:36.
Prigent K, Lasnon C, Ezine E, Janson M, Coudrais N, Joly E, et al. Assessing immune organs on 18F-FDG PET/CT imaging for therapy monitoring of immune checkpoint inhibitors: inter-observer variability, prognostic value and evolution during the treatment course of melanoma patients. Eur J Nucl Med Mol Imaging. 2021.
Şahin E, Elboğa U. Relationship between reticuloendothelial systems’ FDG uptake level and clinicopathological features in patient with invasive ductal breast cancer. Radiol Med. 2017;122:785–92.
Bang J-I, Yoon H-J, Kim BS. Clinical utility of FDG uptake within reticuloendothelial system on F-18 FDG PET/CT for prediction of tumor recurrence in breast cancer. PLoS One. 2018;13:e0208861.
Lee JW, Kim SY, Han SW, Lee JE, Lee HJ, Heo NH, et al. [18F]FDG uptake of bone marrow on PET/CT for predicting distant recurrence in breast cancer patients after surgical resection. EJNMMI Res. 2020;10:72.
Lee JW, Lee M-S, Chung IK, Son MW, Cho YS, Lee SM. Clinical implication of FDG uptake of bone marrow on PET/CT in gastric cancer patients with surgical resection. World J Gastroenterol. 2017;23:2385–95.
Yoon H-J, Kim BS, Moon CM, Yoo J, Lee KE, Kim Y. Prognostic value of diffuse splenic FDG uptake on PET/CT in patients with gastric cancer. PLoS One. 2018;13:e0196110.
Lee JW, Choi JS, Lyu J, Lee SM. Prognostic significance of 18F-fluorodeoxyglucose uptake of bone marrow measured on positron emission tomography in patients with small cell lung cancer. Lung Cancer. 2018;118:41–7.
Jeong H, Hwang I, Kang SH, Shin HC, Kwon SY. Tumor-associated macrophages as potential prognostic biomarkers of invasive breast cancer. J Breast Cancer. 2019;22:38–51.
Hashemi V, Maleki LA, Esmaily M, Masjedi A, Ghalamfarsa G, Namdar A, et al. Regulatory T cells in breast cancer as a potent anti-cancer therapeutic target. Int Immunopharmacol. 2020;78:106087.
Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol. 2020;5.
Seban R-D, Champion L, Schwartz LH, Dercle L. Spleen glucose metabolism on [18F]-FDG PET/CT: a dynamic double-edged biomarker predicting outcome in cancer patients. Eur J Nucl Med Mol Imaging. 2021.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93–9.
Cserni G, Chmielik E, Cserni B, Tot T. The new TNM-based staging of breast cancer. Virchows Arch. 2018;472:697–703.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860–7.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn H-J, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47.
Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54.
Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6.
Viallon V, Latouche A. Discrimination measures for survival outcomes: connection between the AUC and the predictiveness curve. Biom J. 2011;53:217–36.
Empereur-Mot C, Guillemain H, Latouche A, Zagury J-F, Viallon V, Montes M. Predictiveness curves in virtual screening. J Cheminform. 2015;7:52.
Latouche A, Allignol A, Beyersmann J, Labopin M, Fine JP. A competing risks analysis should report results on all cause-specific hazards and cumulative incidence functions. J Clin Epidemiol. 2013;66:648–53.
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria: 2020; Available from: https://www.R-project.org
Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys. 2012;83:878–86.
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50.
Annaratone L, Cascardi E, Vissio E, Sarotto I, Chmielik E, Sapino A, et al. The multifaceted nature of tumor microenvironment in breast carcinomas. Pathobiology. 2020;87:125–42.
Christmas BJ, Rafie CI, Hopkins AC, Scott BA, Ma HS, Cruz KA, et al. Entinostat converts immune-resistant breast and pancreatic cancers into checkpoint-responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol Res. 2018;6:1561–77.
Seban R-D, Assié J-B, Giroux-Leprieur E, Massiani M-A, Soussan M, Bonardel G, et al. Association of the metabolic score using baseline FDG-PET/CT and dNLR with immunotherapy outcomes in advanced NSCLC patients treated with first-line pembrolizumab. Cancers. Multidisciplinary Digital Publishing Institute; 2020;12:2234.
Tylski P, Stute S, Grotus N, Doyeux K, Hapdey S, Gardin I, et al. Comparative assessment of methods for estimating tumor volume and standardized uptake value in (18)F-FDG PET. J Nucl Med. 2010;51:268–76.
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Annals of Oncology Elsevier. 2019;30:1194–220.
McLemore LE, Janakiram M, Albanese J, Shapiro N, Lo Y, Zang X, et al. An immunoscore using PD-L1, CD68, and tumor-infiltrating lymphocytes (TILs) to predict response to neoadjuvant chemotherapy in invasive breast cancer. Appl Immunohistochem Mol Morphol. 2018;26:611–9.
Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3:326–32.
Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018;24:1852–8.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the design of the work or the acquisition, analysis, or interpretation of data; revised it critically for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Material preparation, data collection, and analysis were performed by R-D. Seban, A. Latouche, N. Deleval, F-C. Bidard, and L. Champion. The first draft of the manuscript was written by R-D Seban, F-C. Bidard, and L. Champion. All authors commented on previous versions of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration.
Consent to participate
For this type of study (retrospective), formal consent is not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - General
Supplementary information
ESM 1
(DOCX 2300 kb)
Ackowledgements
We would like to thank Marie-Laure TANGUY for her help (statistical analysis).
Rights and permissions
About this article
Cite this article
Seban, RD., Rouzier, R., Latouche, A. et al. Total metabolic tumor volume and spleen metabolism on baseline [18F]-FDG PET/CT as independent prognostic biomarkers of recurrence in resected breast cancer. Eur J Nucl Med Mol Imaging 48, 3560–3570 (2021). https://doi.org/10.1007/s00259-021-05322-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05322-2