Abstract
Purpose
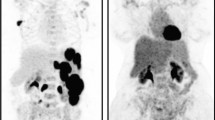
In an Asian international multicenter phase II trial conducted in patients with peripheral T-cell lymphoma (PTCL), [F-18]FDG-PET/CT was used for evaluation of the therapeutic response. Standardization of the PET/CT scanners was necessary before patient enrollment. We therefore standardized the scanners by phantom tests based on the profile approved by the Quantitative Imaging Biomarkers Alliance (QIBA) of Radiological Society of North America (RSNA).
Materials and methods
The tests were conducted on 12 scanners in 12 facilities in compliance with the QIBA Profile and used National Electrical Manufacturers Association (NEMA) International Electrotechnical Commission (IEC) body phantoms. We measured three parameters (standardized uptake value [SUV], resolution and noise) and adjusted the imaging parameter values. The indexes recommended in the Japanese Society of Nuclear Medicine (JSNM) guideline were also evaluated.
Results
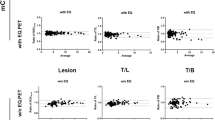
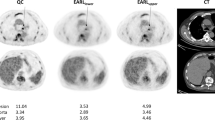
In a total of 12 facilities, 6 facilities required no change in imaging conditions and 6 facilities required changes in imaging parameters. After revision, the three measurements (SUV, resolution and noise) met QIBA criteria at all sites, but 10 of the 12 scanners did not meet JSNM criteria.
Conclusion
We standardized imaging conditions using phantoms as required in the RSNA-QIBA profile for response evaluation by [F-18]FDG PET/CT images in a multicenter study.

Similar content being viewed by others
References
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
Armitage JO, Vose JM, Weisenburger DD. Towards understanding the peripheral T-cell lymphomas. Ann Oncol. 2004;15:1447–9.
Rodriguez-Abreu D, Filho VB, Zucca E. Peripheral T-cell lymphomas, unspecified (or not otherwise specified): a review. Hematol Oncol. 2008;26:8–20.
Chihara D, Fanale MA, Miranda RN, Noorani M, Westin JR, Nastoupil LJ, et al. The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma. Br J Haematol. 2017;176:750–8.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
Sargent DJ, Rubinstein L, Schwartz L, Dancey JE, Gatsonis C, Dodd LE, et al. Validation of novel imaging methodologies for use as cancer clinical trial end-points. Eur J Cancer. 2009;45:290–9.
Meyer CR, Armato SG, Fenimore CP, McLennan G, Bidaut LM, Barboriak DP, et al. Quantitative imaging to assess tumor response to therapy: common themes of measurement, truth data, and error sources. Transl Oncol. 2009;2:198–210.
Buckler AJ, Bresolin L, Dunnick NR, Sullivan DC. A collaborative enterprise for multi-stakeholder participation in the advancement of quantitative imaging. Radiology. 2011;258:906–14.
Buckler AJ, Bresolin L, Dunnick NR, Sullivan DC, Aerts HJ, Bendriem B, et al. Quantitative imaging test approval and biomarker qualification: interrelated but distinct activities. Radiology. 2011;259:875–84.
Sullivan DC, Obuchowski NA, Kessler LG, Raunig DL, Gatsonis C, Huang EP, et al. Metrology standards for quantitative imaging biomarkers. Radiology. 2015;277:813–25.
O'Connor JP, Aboagye EO, Adams JE, Aerts HJ, Barrington SF, Beer AJ, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14:169–86.
Quantitative Imaging Biomarkers Alliance. Radiological Society of North America. https://www.rsna.org/QIBA/
FDG-PET, CT,. Technical Committee. FDG-PET, CT,. as an Imaging Biomarker Measuring Response to Cancer Therapy, Quantitative Imaging Biomarkers Alliance, Version 1.11, Publicly Reviewed Version. QIBA, November 10, 2016. Available from: https://www.rsna.org/QIBA/
Fukukita H, Suzuki K, Matsumoto K, Terauchi T, Daisaki H, Ikari Y, et al. Japanese guideline for the oncology FDG-PET/CT data acquisition protocol: synopsis of Version 2.0. Ann Nucl Med. 2014;28:693–705.
Hirano S, Kobayashi Y. Cytotoxic effects of S-(dimethylarsino)-glutathione: a putative intermediate metabolite of inorganic arsenicals. Toxicology. 2006;227:45–52.
Matulis SM, Morales AA, Yehiayan L, Croutch C, Gutman D, Cai Y, et al. Darinaparsin induces a unique cellular response and is active in an arsenic trioxide-resistant myeloma cell line. Mol Cancer Ther. 2009;8:1197–206.
Hosein PJ, Craig MD, Tallman MS, Boccia RV, Hamilton BL, Lewis JJ, et al. A multicenter phase II study of darinaparsin in relapsed or refractory Hodgkin's and non-Hodgkin's lymphoma. Am J Hematol. 2012;87:111–4.
Ravi D, Bhalla S, Gartenhaus RB, Crombie J, Kandela I, Sharma J, et al. The novel organic arsenical darinaparsin induces MAPK-mediated and SHP1-dependent cell death in T-cell lymphoma and Hodgkin lymphoma cells and human xenograft models. Clin Cancer Res. 2014;20:6023–33.
Lee YS, Kim JS, Kim KM, Kang JH, Lim SM, Kim HJ. Performance measurement of PSF modeling reconstruction (True X) on Siemens Biograph TruePoint TrueV PET/CT. Ann Nucl Med. 2014;28:340–8.
van der Vos CS, Koopman D, Rijnsdorp S, Arends AJ, Boellaard R, van Dalen JA, et al. Quantification, improvement, and harmonization of small lesion detection with state-of-the-art PET. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):4–16.
Daisaki H, Tateishi U, Terauchi T, Tatsumi M, Suzuki K, Shimada N, et al. Standardization of image quality across multiple centers by optimization of acquisition and reconstruction parameters with interim FDG-PET/CT for evaluating diffuse large B cell lymphoma. Ann Nucl Med. 2013;27:225–32.
Acknowledgements
We gratefully appreciate the cooperation of the following members and institutions, JoonYoung Choi (Samsung Medical Center), SeokKi Kim (National Cancer Center), Jin Suck Ryu, JeongSu Oh (Asan Medical Center), MiJin Yun (Severance Hospital), Byeong-Il Kim (Korea Cancer Center Hospital), JungJun Min, SeongYoung Kwon (Chonnam National University Hwasun Hospital), Ruoh-Fang Yen (National Taiwan University Hospital), Wen-Sheng Huang, Bang-Hung Yang (Taipei Veterans General Hospital), Chien Lin, Wen-Cheng Huang (Chang Gung Memorial Hospital Linkou), Te-Chun Hsieh (China Medical University Hospital), Nan-Tsing Chiu (National Cheng Kung University Hospital), and Yang Kwok Wai, Michael (Hong Kong Integrated Oncology Centre). We have also been given helpful suggestions by Edward Jackson, PhD and Alexander Guimaraes, MD, PhD, Steering Committee, Quantitative Imaging Biomarkers Alliance (QIBA), Radiological Society of North America (RSNA).
Funding
No funding was received by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
About this article
Cite this article
Bae, H., Tsuchiya, J., Okamoto, T. et al. Standardization of [F-18]FDG PET/CT for response evaluation by the Radiologic Society of North America-Quantitative Imaging Biomarker Alliance (RSNA-QIBA) profile: preliminary results from the Japan-QIBA (J-QIBA) activities for Asian international multicenter phase II trial. Jpn J Radiol 36, 686–690 (2018). https://doi.org/10.1007/s11604-018-0780-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-018-0780-x