Abstract
Purpose
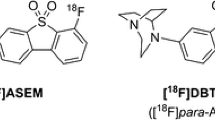
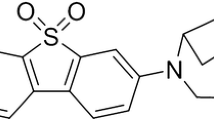
Positron emission tomography (PET) radioligands specific to α7 nicotinic acetylcholine receptors (nAChRs) afford in vivo imaging of this receptor for neuropathologies such as Alzheimer’s disease, schizophrenia, and substance abuse. This work aims to characterize the kinetic properties of an α7-nAChR-specific radioligand, 7-(1,4-diazabicyclo[3.2.2]nonan-4-yl)-2-[18F]-fluorodibenzo[b,d]thiophene 5,5-dioxide ([18F]DBT-10), in nonhuman primates.
Methods
[18F]DBT-10 was produced via nucleophilic substitution of the nitro-precursor. Four Macaca mulatta subjects were imaged with [18F]DBT-10 PET, with measurement of [18F]DBT-10 parent concentrations and metabolism in arterial plasma. Baseline PET scans were acquired for all subjects. Following one scan, ex vivo analysis of brain tissue was performed to inspect for radiolabeled metabolites in brain. Three blocking scans with 0.69 and 1.24 mg/kg of the α7-nAChR-specific ligand ASEM were also acquired to assess dose-dependent blockade of [18F]DBT-10 binding. Kinetic analysis of PET data was performed using the metabolite-corrected input function to calculate the parent fraction corrected total distribution volume (V T/f P).
Results
[18F]DBT-10 was produced within 90 min at high specific activities of 428 ± 436 GBq/μmol at end of synthesis. Metabolism of [18F]DBT-10 varied across subjects, stabilizing by 120 min post-injection at parent fractions of 15–55 %. Uptake of [18F]DBT-10 in brain occurred rapidly, reaching peak standardized uptake values (SUVs) of 2.9–3.7 within 30 min. The plasma-free fraction was 18.8 ± 3.4 %. No evidence for radiolabeled [18F]DBT-10 metabolites was found in ex vivo brain tissue samples. Kinetic analysis of PET data was best described by the two-tissue compartment model. Estimated V T/f P values were 193–376 ml/cm3 across regions, with regional rank order of thalamus > frontal cortex > striatum > hippocampus > occipital cortex > cerebellum > pons. Dose-dependent blockade of [18F]DBT-10 binding by structural analog ASEM was observed throughout the brain, and occupancy plots yielded a V ND/f P estimate of 20 ± 16 ml/cm3.
Conclusion
These results demonstrate suitable kinetic properties of [18F]DBT-10 for in vivo quantification of α7-nAChR binding in nonhuman primates.







Similar content being viewed by others
References
Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther 2013;137:22–54.
Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 2009;89:73–120.
de Jonge WL, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol 2007;151:915–29.
Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the α7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther 2009;122:302–11. doi:10.1016/j.pharmthera.2009.03.009.
Levin ED. α7-Nicotinic receptors and cognition. Curr Drug Targets 2012;13:602–6.
Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol 2013;86:1122–32. doi:10.1016/j.bcp.2013.06.031.
Hernandez CM, Dineley KT. α7 nicotinic acetylcholine receptors in Alzheimer’s disease: neuroprotective, neurotrophic or both? Curr Drug Targets 2012;13:613–22.
Philip NS, Carpenter LL, Tyrka AR, Price LH. The nicotinic acetylcholine receptor as a target for antidepressant drug development. Sci World J 2012;2012.
Kamens H, Andersen J, Picciotto M. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology 2010;208:613–26. doi:10.1007/s00213-009-1759-1.
Brunzell DH, McIntosh JM, Papke RL. Diverse strategies targeting alpha7 homomeric and alpha6beta2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Ann N Y Acad Sci 2014;1327:27–45. doi:10.1111/nyas.12421.
Brust P, Peters D, Deuther-Conrad W. Development of radioligands for the imaging of α7 nicotinic acetylcholine receptors with positron emission tomography. Curr Drug Targets 2012;13:594–601.
Ding M, Ghanekar S, Elmore CS, Zysk JR, Werkheiser JL, Lee CM, et al. [(3)H]Chiba-1001(methyl-SSR180711) has low in vitro binding affinity and poor in vivo selectivity to nicotinic alpha-7 receptor in rodent brain. Synapse 2012;66:315–22. doi:10.1002/syn.21513.
Hashimoto K, Nishiyama S, Ohba H, Matsuo M, Kobashi T, Takahagi M, et al. [11C]CHIBA-1001 as a novel PET ligand for alpha7 nicotinic receptors in the brain: a PET study in conscious monkeys. PLoS One 2008;3:e3231. doi:10.1371/journal.pone.0003231.
Ishikawa M, Sakata M, Toyohara J, Oda K, Ishii K, Wu J, et al. Occupancy of alpha7 nicotinic acetylcholine receptors in the brain by tropisetron: a positron emission tomography study using [(11)C]CHIBA-1001 in healthy human subjects. Clin Psychopharmacol Neurosci 2011;9:111–6. doi:10.9758/cpn.2011.9.3.111.
Toyohara J, Sakata M, Wu J, Ishikawa M, Oda K, Ishii K, et al. Preclinical and the first clinical studies on [11C]CHIBA-1001 for mapping alpha7 nicotinic receptors by positron emission tomography. Ann Nucl Med 2009;23:301–9. doi:10.1007/s12149-009-0240-x.
Horti AG, Gao Y, Kuwabara H, Wang Y, Abazyan S, Yasuda RP, et al. 18F-ASEM, a radiolabeled antagonist for imaging the α7-nicotinic acetylcholine receptor with PET. J Nucl Med 2014;55:672–7. doi:10.2967/jnumed.113.132068.
Wong DF, Kuwabara H, Pomper M, Holt DP, Brasic JR, George N, et al. Human brain imaging of α7 nAChR with [(18)F]ASEM: a new PET radiotracer for neuropsychiatry and determination of drug occupancy. Mol Imaging Biol 2014;16:730–8. doi:10.1007/s11307-014-0779-3.
Teodoro R, Scheunemann M, Deuther-Conrad W, Wenzel B, Fasoli FM, Gotti C, et al., A promising PET tracer for imaging of α7 nicotinic acetylcholine receptors in the brain: desing, synthesis, and in vivo evaluation of a dibenzothiophene-based radioligand. Molecules; In Press. doi:10.3390/molecules201018387.
Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol 2000;27:627–30.
Lin S-F, Labaree D, Chen M-K, Holden D, Gallezot J-D, Kapinos M, et al. Further evaluation of [11C]MP-10 as a radiotracer for phosphodiesterase 10A: PET imaging study in rhesus monkeys and brain tissue metabolite analysis. Synapse 2015;69:86–95. doi:10.1002/syn.21792.
Sandiego CM, Weinzimmer D, Carson RE. Optimization of PET-MR registrations for nonhuman primates using mutual information measures: a Multi-Transform Method (MTM). Neuroimage 2013;64:571–81. doi:10.1016/j.neuroimage.2012.08.051.
Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, et al. The INIA19 Template and NeuroMaps Atlas for primate brain image parcellation and spatial normalization. Front Neuroinform 2012;6:27. doi:10.3389/fninf.2012.00027.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–9. doi:10.1038/sj.jcbfm.9600493.
Gunn RN, Gunn SR, Cunningham VJ. Positron emission tomography compartmental models. J Cereb Blood Flow Metab 2001;21:635–52. doi:10.1097/00004647-200106000-00002.
Hurvich CM, Tsai C-L. Regression and time series model selection in small samples. Biometrika 1989;76:297–307.
Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab 2002;22:1271–81. doi:10.1097/00004647-200210000-00015.
Cunningham VJ, Rabiner EA, Slifstein M, Laruelle M, Gunn RN. Measuring drug occupancy in the absence of a reference region: the Lassen plot re-visited. J Cereb Blood Flow Metab 2010;30:46–50. doi:10.1038/jcbfm.2009.190.
Han ZY, Zoli M, Cardona A, Bourgeois JP, Changeux JP, Le Novère N. Localization of [3H]nicotine, [3H]cytisine, [3H]epibatidine, and [125I]alpha-bungarotoxin binding sites in the brain of Macaca mulatta. J Comp Neurol 2003;461:49–60. doi:10.1002/cne.10659.
Gallezot JD, Weinzimmer D, Nabulsi N, Lin SF, Fowles K, Sandiego C, et al. Evaluation of [(11)C]MRB for assessment of occupancy of norepinephrine transporters: studies with atomoxetine in non-human primates. Neuroimage 2011;56:268–79. doi:10.1016/j.neuroimage.2010.09.040.
Kulak JM, Carroll FI, Schneider JS. [125I]Iodomethyllycaconitine binds to α7 nicotinic acetylcholine receptors in monkey brain. Eur J Neurosci 2006;23:2604–10. doi:10.1111/j.1460-9568.2006.04804.x.
Acknowledgments
This work was supported by NIH T32 DA022975. We thank the staff at the Yale PET Center for their expert technical assistance in support of this work. This publication was made possible by CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Yale University Institutional Animal Care and Use Committee.
Rights and permissions
About this article
Cite this article
Hillmer, A.T., Zheng, MQ., Li, S. et al. PET imaging evaluation of [18F]DBT-10, a novel radioligand specific to α7 nicotinic acetylcholine receptors, in nonhuman primates. Eur J Nucl Med Mol Imaging 43, 537–547 (2016). https://doi.org/10.1007/s00259-015-3209-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3209-0