Abstract
Purpose
Optical imaging is emerging as a powerful tool for the noninvasive imaging of the biological processes in living subjects. This study aimed to investigate whether optical imaging of integrin αvβ3 and vascular endothelial growth factor (VEGF) expression can serve as sensitive biomarkers for tumor early response to antiangiogenic therapy.
Methods
We synthesized two near-infrared fluorescence (NIRF) imaging agents, CF680R-3PRGD2 and CF750-BevF(ab')2, which were designed to specifically bind to integrin αvβ3 and VEGF, respectively. The ability of optical imaging using the two imaging agents for early monitoring the antiangiogenic effect of sunitinib was evaluated.
Results
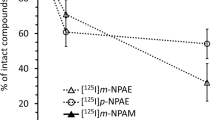
CF680R-3PRGD2 and CF750-BevF(ab')2 specifically bound to their respective targets in vitro and in HT-29 tumor-bearing nude mice. Sunitinib treatment led to significantly decreased tumor uptake of CF680R-3PRGD2 (e.g., 7.47 ± 1.62 % vs. 4.24 ± 0.16 % on day 4; P < 0.05) and CF750-BevF(ab')2 (e.g., 7.43 ± 2.43 % vs. 4.04 ± 1.39 % on day 2; P < 0.05) in vivo. Immunofluorescence staining and an enzyme-linked immunosorbent assay confirmed that sunitinib-induced changes in tumor uptake of CF680R-3PRGD2 and CF750-BevF(ab')2 were correlated with changes in the levels of integrin αvβ3 and VEGF. Radiobiodistribution of 99mTc-3PRGD2 and 125I-BevF(ab')2, the radiocounterparts of CF680R-3PRGD2 and CF750-BevF(ab')2, respectively, also validated optical imaging results.
Conclusion
Longitudinal monitoring of tumor integrin αvβ3 and VEGF expression could be used as early biomarkers for tumor response to antiangiogenic therapy. This strategy may facilitate the development of new antiangiogenic drugs, and be used for elucidation of the underlying mechanisms of therapies involving the integrin and the VEGF signaling pathway.







Similar content being viewed by others
References
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Eliceiri BP, Cheresh DA. The role of alphav integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–30.
Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alphavbeta3 for angiogenesis. Science. 1994;264:569–71.
Kumar CC. Integrin alphavbeta3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4:123–31.
Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39 Suppl 1:S126–38.
Haubner R, Beer AJ, Wang H, Chen X. Positron emission tomography tracers for imaging angiogenesis. Eur J Nucl Med Mol Imaging. 2010;37 Suppl 1:S86–103.
Liu Z, Wang F. Development of RGD-based radiotracers for tumor imaging and therapy: translating from bench to bedside. Curr Mol Med. 2013;13:1–19.
Liu S. Radiolabeled cyclic RGD peptides as integrin alphavbeta3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009;20:2199–213.
Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–73.
Papaetis GS, Syrigos KN. Sunitinib: a multitargeted receptor tyrosine kinase inhibitor in the era of molecular cancer therapies. BioDrugs. 2009;23:377–89.
Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–96.
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9.
Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31.
Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189–204.
Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med. 2006;47:793–6.
Nagengast WB, de Korte MA, Oude Munnink TH, Timmer-Bosscha H, den Dunnen WF, Hollema H, et al. 89Zr-bevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J Nucl Med. 2010;51:761–7.
van der Bilt AR, Terwisscha van Scheltinga AG, Timmer-Bosscha H, Schroder CP, Pot L, Kosterink JG, et al. Measurement of tumor VEGF-A levels with 89Zr-bevacizumab PET as an early biomarker for the antiangiogenic effect of everolimus treatment in an ovarian cancer xenograft model. Clin Cancer Res. 2012;18:6306–14.
Chang AJ, Sohn R, Lu ZH, Arbeit JM, Lapi SE. Detection of rapalog-mediated therapeutic response in renal cancer xenografts using 64Cu-bevacizumab immunoPET. PLoS One. 2013;8:e58949.
Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol. 2002;13:603–8.
Nagengast WB, Lub-de Hooge MN, Oosting SF, den Dunnen WF, Warnders FJ, Brouwers AH, et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res. 2011;71:143–53.
Battle MR, Goggi JL, Allen L, Barnett J, Morrison MS. Monitoring tumor response to antiangiogenic sunitinib therapy with 18F-fluciclatide, an 18F-labeled alphavbeta3-integrin and alphavbeta5-integrin imaging agent. J Nucl Med. 2011;52:424–30.
Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR, Gambhir SS, et al. Quantitative PET imaging of tumor integrin alphavbeta3 expression with 18F-FRGD2. J Nucl Med. 2006;47:113–21.
Liu Z, Jia B, Shi J, Jin X, Zhao H, Li F, et al. Tumor uptake of the RGD dimeric probe 99mTc-G(3)-2P(4)-RGD2 is correlated with integrin alphavbeta3 expressed on both tumor cells and neovasculature. Bioconjug Chem. 2010;21:548–55.
Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–8.
Jia B, Liu Z, Zhu Z, Shi J, Jin X, Zhao H, et al. Blood clearance kinetics, biodistribution, and radiation dosimetry of a kit-formulated integrin alphavbeta3-selective radiotracer 99mTc-3PRGD 2 in non-human primates. Mol Imaging Biol. 2011;13:730–6.
Liu Z, Jia B, Zhao H, Chen X, Wang F. Specific targeting of human integrin alphavbeta3 with 111In-labeled Abegrin in nude mouse models. Mol Imaging Biol. 2011;13:112–20.
Liu Z, Huang J, Dong C, Cui L, Jin X, Jia B, et al. 99mTc-labeled RGD-BBN peptide for small-animal SPECT/CT of lung carcinoma. Mol Pharm. 2012;9:1409–17.
Liu Z, Liu S, Niu G, Wang F, Liu S, Chen X. Optical imaging of integrin alphavbeta3 expression with near-infrared fluorescent RGD dimer with tetra(ethylene glycol) linkers. Mol Imaging. 2010;9:21–9.
Liu Z, Niu G, Shi J, Liu S, Wang F, Liu S, et al. 68Ga-labeled cyclic RGD dimers with Gly3 and PEG4 linkers: promising agents for tumor integrin alphavbeta3 PET imaging. Eur J Nucl Med Mol Imaging. 2009;36:947–57.
Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95.
Jin ZH, Furukawa T, Claron M, Boturyn D, Coll JL, Fukumura T, et al. Positron emission tomography imaging of tumor angiogenesis and monitoring of antiangiogenic efficacy using the novel tetrameric peptide probe 64Cu-cyclam-RAFT-c(−RGDfK-)4. Angiogenesis. 2012;15:569–80.
Sun X, Yan Y, Liu S, Cao Q, Yang M, Neamati N, et al. 18F-FPPRGD2 and 18F-FDG PET of response to Abraxane therapy. J Nucl Med. 2011;52:140–6.
Janjigian YY, Viola-Villegas N, Holland JP, Divilov V, Carlin SD, Gomes-DaGama EM, et al. Monitoring afatinib treatment in HER2-positive gastric cancer with 18F-FDG and 89Zr-trastuzumab PET. J Nucl Med. 2013;54:936–43.
Chen X, Conti PS, Moats RA. In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res. 2004;64:8009–14.
Zhang Y, Hong H, Engle JW, Yang Y, Barnhart TE, Cai W. Positron emission tomography and near-infrared fluorescence imaging of vascular endothelial growth factor with dual-labeled bevacizumab. Am J Nucl Med Mol Imaging. 2012;2:1–13.
Acknowledgments
This work was supported, in part, by the National Natural Science Foundation of China (NSFC) projects (81222019, 81125011, and 81000625), “973” projects (2013CB733802 and 2011CB707703), grants from the Ministry of Science and Technology of China (2011YQ030114, 2012ZX09102301, and 2012BAK25B03), grants from the Ministry of Education of China (31300 and BMU20110263), grants from the Beijing Natural Science Foundation (7132131 and 7132123), and a grant from the Beijing Nova Program (Z121107002512010).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, X., Ma, T., Liu, H. et al. Longitudinal monitoring of tumor antiangiogenic therapy with near-infrared fluorophore-labeled agents targeted to integrin αvβ3 and vascular endothelial growth factor. Eur J Nucl Med Mol Imaging 41, 1428–1439 (2014). https://doi.org/10.1007/s00259-014-2702-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2702-1