Abstract
Purpose
188Re-HEDP is indicated for the treatment of pain in patients with painful osteoblastic bone metastases, including hormone-refractory prostate cancer patients. Efficacy may be improved by adding chemotherapy to the treatment regimen as a radiation sensitizer. The combination of 188Re-HEDP and capecitabine (Xeloda®) was tested in a clinical phase I study.
Methods
Patients with hormone-refractory prostate cancer were treated with capecitabine for 14 days (oral twice daily in a dose escalation regimen with steps of 1/3 of 2,500 mg/m2 per day in cohorts of three to six patients, depending on toxicity). Two days later patients were treated with 37 MBq/kg 188Re-HEDP as an intravenous injection. Six hours after treatment post-therapy scintigraphy was performed. Urine was collected for 8 h post-injection. Follow-up was at least 8 weeks. The primary end-point was to establish the maximum tolerable dose (MTD) of capecitabine when combined with 188Re-HEDP. Secondary end-points included the effect of capecitabine on the biodistribution and pharmacokinetics of 188Re-HEDP.
Results
Three patients were treated in the first and second cohorts, each without unacceptable toxicity. One of six patients in the highest cohort experienced unacceptable toxicity (grade 4 thrombopaenia). The MTD proved to be the maximum dose of 2,500 mg/m2 per day capecitabine. No unexpected toxicity occurred. Capecitabine had no effect on uptake or excretion of 188Re-HEDP.
Conclusion
Capecitabine may be safely used in combination with 188Re-HEDP in a dose of 2,500 mg/m2 per day and 37 MBq/kg, respectively. Efficacy will be further studied in a phase II study using these dosages.
Similar content being viewed by others
Introduction
The majority of patients with hormone-refractory prostate cancer have, or will have, osseous metastases in the course of their disease [1]. Treatment with bone-seeking radiopharmaceuticals may be indicated when they experience refractory bone pain at multiple sites. Bone-seeking radiopharmaceuticals decrease pain and improve the patients’ quality of life [2]. This effect may be increased by concomitant use of chemotherapy, used as a radiation sensitizer. Some studies show promising results on the use of chemotherapy as a radiation sensitizer for bone-seeking radiopharmaceuticals, but evidence is still low [3–5]. However, it is clear that multimodality treatment may enhance efficacy and may lead us beyond palliation alone towards improvement of survival [6]. In the present study the combination of the bone-seeking radiopharmaceutical 188Re-HEDP and capecitabine chemotherapy was studied in a phase I setting.
188Re-hydroxyethylidene-1,1-diphosphonic acid (188Re-HEDP) is a relatively new and attractive radiopharmaceutical for the treatment of metastatic bone pain. It has an affinity for skeletal tissue and concentrates in areas of bone turnover secondary to invasion by tumour. As a product of a 188W/188Re generator, it is convenient for clinical therapeutic use, because of on demand use at relatively low costs. The radioisotope, with a half-life of 16.9 h, emits a 155 keV gamma ray (15%) for external imaging and a number of beta particles (\(E_{\beta \;\max } 2.12\;MeV\); \(E_{\beta \;mean} {\text{ }}0.76\;MeV\)) for localized radiotherapy [7, 8]. Therapy with 188Re-HEDP results in symptomatic relief of bone pain in approximately 70–80% of treated patients. 188Re-HEDP is an effective and well-tolerated treatment in the management of metastatic bone pain [9–12].
Capecitabine is a fluoropyrimidine carbamate with antineoplastic activity. It is an orally administered systemic prodrug of 5′-deoxy-5-fluorouridine which is converted to 5-fluorouracil (5-FU) inside the tumour cell by thymidine phosphorylase. 5-FU is metabolized to 5-fluoro-2′-deoxyuridine monophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). These metabolites cause cell injury by two different mechanisms. First, FdUMP and the folate cofactor, N-5-10-methylenetetrahydrofolate, bind to thymidylate synthase to form a covalently bound ternary complex. This binding inhibits the formation of thymidylate from 2′-deoxyuridylate. Thymidylate is the necessary precursor of thymidine triphosphate, which is essential for the synthesis of DNA, so that a deficiency of this compound can inhibit cell division. Second, nuclear transcriptional enzymes can mistakenly incorporate FUTP in place of uridine triphosphate during the synthesis of RNA. This metabolic error can interfere with RNA processing and protein synthesis [13, 14].
5-FU has long been used as a radiation sensitizer. Within a few years of the discovery of 5-FU, radiation sensitization by 5-FU was used in clinical trials. Improved survival and local control with acceptable toxicity profiles were shown in cancers of the oesophagus, anus and rectum [15, 16]. 5-FU chemoradiation schedules were optimized and oral 5-FU analogues that may be substituted for intravenous administration were developed. The sensitizing effects of 5-FU in vitro are maximal when exposure to 5-FU occurs for at least 24 h and up to 48 h after the radiation exposure, supporting the use of continuous infusion or oral 5-FU analogues instead of bolus intravenous administration [17]. Capecitabine (Xeloda®) can be administered orally, resulting in continuous high levels of active FU. In contrast to 5-FU, capecitabine has an improved therapeutic to toxicity index, because it is metabolized to cytotoxic 5-FU in the target cell by way of thymidine phosphorylase. Measuring the activity of thymidine phosphorylase in normal and cancerous prostatic tissue has shown significantly higher levels in cancerous tissue, making capecitabine a potentially more active agent against prostate cancer than 5-FU [18, 19]. Compared to oral 5-FU prodrugs, protracted venous infusion is costly, inconvenient and has a risk of central line maintenance. Capecitabine is currently used as a radiation sensitizer in several cancer types, like advanced colorectal cancer and pancreatic cancer [16, 20].
Radiation sensitization by capecitabine in hormone-refractory prostate cancer patients has never been described. Like in other cancer types, it could lead to enhancement of the radiation effect in prostate cancer [14]. This radiation-sensitizing effect of capecitabine could enhance the palliative effect of treatment with the bone-seeking radiopharmaceutical 188Re-HEDP. The primary aim of this phase I study was to establish the safety and toxicity profile and to determine the maximum tolerated dose (MTD) of capecitabine combined with 188Re-HEDP. Secondary end-points included the effect of capecitabine on the biodistribution and pharmacokinetics of 188Re-HEDP.
Materials and methods
Study population
Patients with histologically documented adenocarcinoma of the prostate, progressive hormone-refractory disease and more than one painful bone metastasis (99mTc-HDP scintigraphy within 8 weeks prior to screening) were included in this open-label prospective phase I study. Other inclusion criteria were a Karnofsky performance score of at least 60%, life expectancy of at least 3 months, age of at least 18 years and the ability to understand and willingness to sign an informed consent document. Patients receiving bisphosphonate therapy had to discontinue their treatment for at least 2 weeks prior to study entry, and patients under luteinizing hormone-releasing hormone (LH-RH) agonists (and/or anti-androgens) had to continue their treatment. Patients with pathological long-bone fractures or clinically evident spinal cord compression and patients with known malignancies other than prostate cancer (not including basal cell carcinoma of the skin) were excluded. Other exclusion criteria were chemotherapy (including Estracyt®) within 6 weeks prior to screening; prior treatment with systemic radiotherapeutic bone agents within 3 months (6 months for 89Sr); receipt of any other investigational drug within 4 weeks of study entry; previous hemi-body external radiation therapy (for >25% of the bone marrow within 6 months); concomitant treatment with interferon-alfa, allopurinol, sorivudine and folinic acid; clinically significant bleeding disorders; disseminated intravascular coagulation; hypersensitivity to phosphonate compounds or 5-FU; known deficiency of dihydropyrimidine dehydrogenase; concurrent illnesses or treatments that might preclude study completion; active CNS or epidural brain metastasis; absolute neutrophil count (ANC) < 2 × 109/l; platelet count < 150 × 109/l; haemoglobin < 6.0 mmol/l; serum creatinine clearance < 50 ml/min (Cockroft and Gault); bilirubin > 1.5 upper limit of normal; aspartate aminotransferase (AST)/alanine aminotransferase (ALT) > 2.5 upper limit of normal; or total prostate-specific antigen (PSA) < 5 ng/ml. The study was approved by the local Ethics Committee, and written informed consent was obtained from all patients.
188Re-HEDP
188Re was obtained from an alumina-based 188W/188Re-generator on site. The 188W was produced by double-neutron capture of 186 W. Elution of the 188W/188Re-generator with 3 ml normal saline provided solutions of carrier-free 188Re sodium perrhenate (NaReO4). High-performance liquid chromatographic (HPLC) analysis revealed that the 188Re eluate was > 99% perrhenate [7]. 188W/188Re-generators have demonstrated consistently high 188Re yields and low parent breakthrough for periods of at least 2 months.
A HEDP (hydroxyethylidene-1,1-diphosphonic acid) vial contained exactly 15 mg of Na2HEDP, 4.5 mg Sn2Cl-2H2O, 4.0 mg of gentisinic acid 98% and 0.1 mg NH4ReO4. A second vial contained a sodium acetate trihydrate solution of 41 mg/ml in aqua distillate (0.3 M). After preparation the HEDP kit was immediately stored at −20°C. To make 188Re-HEDP, 1.0 ml NH4ReO4 solution (containing 0.01–0.1 mg of NH4ReO4) and 1 ml 188ReO4− was added to the kit vial. The whole mixture was heated for 20 min in a heating block at 120°C and allowed to cool to room temperature for 10 min. Another 1 ml of 0.3 M sodium acetate trihydrate solution was added to adjust the pH range to 5–6. The radiochemical purity of 188Re-HEDP was determined by the instant thin-layer chromatographic (ITLC) technique [7].
Treatment
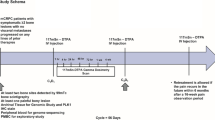
Cohorts of three successive patients were treated with a combination of capecitabine (Xeloda®; Roche, Woerden, The Netherlands) and 188Re-HEDP. Capecitabine treatment started 48 h before 188Re-HEDP administration. The first cohort was treated with 1/3 × 2,500 mg/m2 per day capecitabine, followed by a weight-related dose of 37 MBq/kg body weight 188Re-HEDP. Capecitabine was administered in twice daily doses for 14 days. Escalation of administered doses of capecitabine were implemented in increments of 1/3 × 2,500 mg/m2 per day to a maximum dose of 2,500 mg/m2 per day capecitabine (the maximum recommended dose for metastatic breast and colorectal cancer). Follow-up lasted for 8 weeks with weekly blood samples and 4-weekly history taken and physical exam.
Patients were hospitalized for 8 h after 188Re-HEDP administration. Urine was collected during the 8 h following injection of 188Re-HEDP. Whole-body images were captured with a dual-head gamma camera at 6 h post-injection (anterior and posterior; 10% energy window around the peak of 155 keV, medium energy collimator, scan speed 6 cm/min).
Analysis
If one dose limiting toxicity (DLT) would occur in the cohort of three patients then the cohort would increase to six patients. If a maximum of one of six patients would have had a DLT then the next cohort would have been tested. If at least two of six patients would have had a DLT then the MTD (i.e. the dosage level of the previous cohort) would have been reached. At least six patients were treated in the final MTD group.
Any of the following events which were considered possibly or probably related to the administration of capecitabine, 188Re-HEDP or a combination of those were considered a DLT during the 8 weeks of follow-up [using National Cancer Institute (NCI) Common Terminology for Adverse Events version 3.0]: grade 3–4 neutropaenic infection (ANC < 1.0 × 109/l) with fever > 38.3°C; grade 4 neutropaenia lasting > 7 days; grade 4 thrombocytopaenia (platelet count < 25 × 109/l); grade 3 thrombocytopaenia lasting for > 7 days; any non-haematological grade 3 or 4 toxicity possibly related to study medication; grade 3–5 nausea, vomiting, mucositis, fatigue, tearing, nail disturbance, alopecia or diarrhoea; any life-threatening event possibly related to the study drug. Disease progression was not considered a DLT event.
Secondary end-points were the evaluation of pharmacokinetics and biodistribution of 188Re-HEDP when combined with capecitabine. Post-treatment scintigraphy was compared with pre-treatment 99mTc-HDP scintigraphy performed within 8 weeks of study entry. Regions of interest (ROI analysis) were used to calculate lesion to normal bone ratios (geometric mean corrected for soft tissue uptake and background). The mean counts per pixel calculated over three metastases were used as lesion value; the mean counts per pixel for a ROI over the femur was used as normal bone value. The same ROIs were used for post- and pre-treatment scans in the same patient.
Urinary excretion of activity was measured during the first 8 h after treatment and compared to literature values of 188Re-HEDP as monotherapy. Pooled urine samples were collected from 0–4 h and 4–8 h following 188Re-HEDP administration. The amount of activity in these samples was determined by measurement of 15-ml, non-diluted samples with a dose calibrator. For comparison with the administered activity, the exact injected dose was determined by measurement of the syringe before and after administration. This procedure enabled determination of the amount of activity excreted and, as a corollary, the relative amount of activity retained within the body.
The extent of osteoblastic bone disease was determined using the bone scan index as described by Blake et al. [21]. The skeleton was divided into four anatomical regions: (1) spine and skull, (2) pelvis, (3) shoulder girdle and ribs and (4) extremities. Each region was scored visually on a scale of 0 to 10 for the apparent proportion of skeleton involved. Scores for each region were summed, and the sum was normalized to a scale of 0 to 100 as an index for the extent of skeletal involvement.
Statistical methods
Descriptive statistics (n, mean, SD, minimum and maximum) were calculated for quantitative variables; frequency counts by category were determined for qualitative variables.
The bone scan index was correlated with the excreted activity in urine using Pearson’s bivariate correlation coefficient for continuous variables (R). The hypothesis ‘no correlation’ versus the alternative hypothesis ‘significant correlation’ was tested using Student’s t test (one-tailed).
Results
A total of 17 patients were enrolled in this study. Five patients were excluded after screening because one patient withdrew consent before the start of treatment (because of too many visits to the hospital), three patients had thrombocyte levels < 150 × 109/l and one patient had rapidly progressive disease with a Karnofsky performance score < 60% before the start of treatment. All 12 treated patients were included in the safety and toxicity analysis (the primary end-point). All 12 treated patients were Caucasian with a mean age of 70 years (range: 60–83) (Table 1).
Among the 12 patients who entered the treatment period, 11 patients completed both the treatment period and the study course (9 weeks). One patient (patient 15) did not complete the last visit because of progressive disease. Adverse events (> grade 1 or > 1 patient) are listed in Table 2. The first two cohorts were treated without unacceptable adverse events. Five of six patients in the last cohort were treated without unacceptable adverse events. One patient (treated with 37 MBq/kg 188Re-HEDP and 2,500 mg/m2 per day capecitabine) suffered from progressive complaints of fatigue (grade 3), which made it impossible to comply with the last follow-up visit. Laboratory values showed a progressive increase of PSA and alkaline phosphatase together with a prolonged and unrecovered bone marrow suppression with anaemia (grade 3), thrombopaenia (grade 4) and leucopaenia/neutropaenia (grade 2). He died of cancer-related events (progressive bone marrow disease) 2 months after the end of the study. Of six patients in the highest cohort he was the only patient who suffered from a DLT. It was probably caused by treatment-related toxicity aggravated by severe progression of disease. The maximum tolerable dose of capecitabine in combination with 37 MBq/kg 188Re-HEDP was therefore 2,500 mg/m2 per day.
After onset of the study treatment, haematological parameters were measured on a weekly basis (Fig. 1) and serum chemistry on a monthly basis (Table 2). All patients experienced an expected temporary decline in platelet count in week 4 (mean ± 1 SD: −61.8 ± 16.5%), 3 weeks after 188Re-HEDP administration, with subsequent recovery (Fig. 1). Also as expected, there was a temporary decline in white blood cell count in week 4 (mean ± 1 SD: −34.4 ± 17.8%), 3 weeks after 188Re-HEDP administration, with recovery thereafter. The temporary increase in mean white blood cell count in week 5 may be due to inter- and intra-patient variations, typically seen in white blood cell count. It was probably not related to the study treatment. Haemoglobin levels were steady throughout the study course with a mean change compared to baseline of −0.8 ± 12.7% 4 weeks after 188Re-HEDP administration and −5.8 ± 11% at the end of the study (Fig. 1).
Grades 1, 2, 3 and 4 haematological toxicity occurred in 6, 3, 0 and 1 of 12 patients, respectively. Creatinine levels were stable throughout the study period for all patients. Clinical adverse events included pain, fatigue, nausea, vomiting, stomatitis and diarrhoea. Most adverse events were probably related to capecitabine and were more frequently encountered in cohorts 2 and 3 (Table 2). An increase in pain happened in four patients, all more than 4 weeks after treatment with initially a good response. These complaints were considered disease progression, not related to study medication. One patient experienced the hand-foot syndrome (grade 2) typically related to capecitabine. The hand-foot syndrome (palmar-plantar erythrodysaesthesia or chemotherapy-induced acral erythema) is characterized by numbness, dysaesthesia, tingling, swelling, pain, erythema, desquamation, blistering and sometimes ulceration (not in this case). This patient proved not to have a dihydropyrimidine dehydrogenase deficiency. He fully recovered.
As mentioned above one serious adverse reaction was observed (grade 4 thrombopaenia), probably related to study treatment (patient 15). Other grade 3 toxicities were probably related to disease progression (pain increase, alkaline phosphatase increase) or other non-related causes (exacerbation of cor pulmonale with pneumonia). The latter patient was hospitalized. He was successfully treated with antibiotics and diuretics. One of the four patients who experienced an increase of bone pain was hospitalized for better medical palliation. These two hospitalizations (serious adverse events) were most probably not related to the study treatment. No suspected unexpected serious adverse reactions (SUSAR) occurred.
Secondary end-points included evaluation of urinary excretion and uptake of 188Re-HEDP in pathological bone lesions. Major protocol deviations leading to exclusion from analysis of secondary end-points were found in two patients. Urine collection after treatment was not complete in these patients. The mean urinary excretion of activity during the first 8 h after injection was 45.7 ± 11.9% (range: 24–60%). As expected no focal activity was visualized outside the skeleton, kidneys and bladder (Fig. 2). So the retained activity was mostly retained in the skeleton. An expected negative correlation was therefore found between urinary excretion of activity and the extent of osteoblastic bone disease (R = −0.83; p = 0.001). The mean bone scan index was 42.7 ± 15.9% (range: 23–75%). Considering skeletal uptake of activity: the lesion to normal bone ratio was 13.4 ± 4.9 for 188Re-HEDP and 14.4 ± 6.8 for 99mTc-HDP.
Efficacy will be further studied in a phase II study using 37 MBq/kg 188Re-HEDP in combination with 2,500 mg/m2 per day. In the present phase I dose escalation study no conclusions were drawn on efficacy.
Discussion
The haematological toxicity profile of 188Re-HEDP in combination with capecitabine (up to 2,500 mg/m2 per day) is comparable to that of monotherapy with 188Re-HEDP. An expected decline in platelet and white blood cell count occurred 3–4 weeks after treatment with subsequent recovery. Additive toxicity was attributed to capecitabine use. One of six patients in the highest cohort experienced unacceptable DLT. This patient had widespread metastatic disease, but was in good clinical condition before treatment (Karnofsky performance score 90%) and was not treated with chemotherapy, nuclear therapy or radiotherapy before study treatment. His PSA was 260 ng/ml with a doubling time of 2 weeks before treatment. After treatment his PSA and alkaline phosphatase further increased, reflecting progressive disease. He experienced grade 4 haematological toxicity (thrombopaenia) and was not able to recover due to progressive bone marrow disease.
188Re-HEDP is currently used to relieve pain in patients with confirmed osteoblastic metastatic bone lesions that enhance on 99mTc-HDP scintigraphy. Experience with 188Re-HEDP in pain reduction is somewhat limited, but results have been quite promising. A number of publications show its efficacy [9–12]. Repeated treatment with 188Re-HEDP with an interval of 8 weeks enhanced pain palliation and improved progression-free and overall survival [11]. Administered doses of around 37 MBq/kg proved to be safe in several studies [10, 11, 22]. Expected bone marrow depression occurred in most cases, but with subsequent recovery. The severity of this depression is related to the bone marrow reserve, which might be compromised as a result of previous treatments or disease progression [22]. Treatment regimens that combine 188Re-HEDP with other modalities have never been tested. The present study shows the safety of 188Re-HEDP combined with capecitabine (up to 2,500 mg/m2 per day).
Carrier-added 188Re-HEDP shows identical chemical characteristics to 186Re-HEDP. The degree of skeletal uptake of 188Re-HEDP correlated with the extent of osteoblastic bone disease. The uptake of 188Re-HEDP in skeletal lesions in the present study (mean ratio: 13.4 ± 4.9) is comparable to pre-treatment skeletal scintigraphy using 99mTc-HDP (mean ratio: 14.4 ± 6.8). These findings correlate with pre-clinical data of 188Re-HEDP in rabbits [7]. However, because of differences in scanning parameters (6 h versus 3 h p.i., energy peak, energy window, scanning speed) a direct comparison has several limitations. The present study was not designed for equivalence testing. Nevertheless, it may be concluded that uptake of 188Re-HEDP in skeletal lesions is sufficient when combined with capecitabine. It is comparable to what may be expected from pre-treatment scintigraphy using 99mTc-HDP.
Like 186Re-HEDP clearance of 188Re-HEDP is exclusively renal, with the remainder of the dose retained in the skeleton. The mean urinary excretion of activity during the first 8 h after injection in the present study was 45.7 ± 11.9% (range: 24–60%). In another study 188Re-HEDP showed a rapid urinary excretion within the first 8 h after therapy, with approximately 41% of the 188Re-HEDP administered being excreted [23]. This is comparable to our data. The large range is attributed to large differences in the extent of metastatic disease. It is unlikely that capecitabine has any effect on urinary excretion or skeletal uptake of 188Re-HEDP. The combination seems feasible.
Capecitabine is indicated as monotherapy in patients with colorectal cancer and breast cancer. It is administered as oral tablets in a dose of 1,250 mg/m2 twice daily for 14 days and 1 week rest in cycles of 3 weeks [14]. Early in vitro studies in human prostate cancer cell lines demonstrated high levels of thymidine phosphorylase inside these cells, necessary for the conversion to active 5-FU [19]. After administration of capecitabine a high anti-tumour effect was found, with a 77% inhibition of growth [24]. The beneficial effect of capecitabine in patients with hormone-refractory prostate cancer was first reported in a patient with advanced disease (multiple bone and liver metastases). Capecitabine was given in a dose of 2,000 mg/m2 per day for 14 days of a 21-day cycle for 6 months. His PSA normalized, the liver size decreased by 7 cm to a normal size and the liver enzymes and alkaline phosphatase also normalized [25]. This finding could not be confirmed in phase II studies using capecitabine as a single agent in hormone-refractory prostate cancer patients [26, 27]. It was however concluded that combined treatment regimens containing capecitabine should be considered, because capecitabine appeared to modulate tumour biology [27].
The results of these early trials indicate that the role of the 5-FU prodrug capecitabine alone or in combination is yet unclear. Evidence is still low. However, prostate cancer cells are sensitive to radiotherapy (both external beam radiotherapy and systemic radionuclide therapy) and potentially to capecitabine [24]. The proven effects of capecitabine as a radiation sensitizer in other cancer types, the lack of data on capecitabine as a radiation sensitizer in advanced prostate cancer and the convenience and toxicity profile of capecitabine make it a good candidate for phase I/II testing in hormone-refractory prostate cancer patients in combination with 188Re-HEDP.
Another agent that may be used in combination with 188Re-HEDP is docetaxel. In contrast to capecitabine, it is not commonly used as a radiation sensitizer and it has some disadvantages in comparison with capecitabine (i.e. intravenous infusion, costly, more side effects). In the present study capecitabine was therefore tested instead of docetaxel. As an anti-tumour agent docetaxel is however more effective in hormone-refractory prostate cancer and it may be used as a radiation sensitizer nevertheless [28]. Moreover, docetaxel and capecitabine may be combined with 188Re-HEDP together. Taxanes were found to upregulate the tumoural activity of thymidine phosphorylase (a critical enzyme for capecitabine activation) and have shown synergistic cytotoxic activity when combined with capecitabine [29, 30]. Docetaxel in combination with capecitabine has already been tested in clinical phase I/II studies with promising results [31, 32]. The docetaxel/capecitabine combination proved to be tolerable and effective. Randomized trials have so far not been conducted.
Capecitabine in combination with 188Re-HEDP proved to be feasible and safe. The next step in the enhancement of efficacy may be docetaxel, capecitabine and 188Re-HEDP as triple therapy in hormone-refractory prostate cancer patients with multiple osseous metastases.
Conclusion
The maximum tolerable dose of capecitabine in combination with 37 MBq/kg 188Re-HEDP is 2,500 mg/m2 per day. The combination is feasible and safe. Efficacy, using the maximum dose, will be tested in a phase II trial.
References
Berthold DR, Sternberg CN, Tannock IF. Management of advanced prostate cancer after first-line chemotherapy. J Clin Oncol 2005;23:8247–52. doi:10.1200/JCO.2005.03.1435.
Lam MG, de Klerk JM, van Rijk PP, Zonnenberg BA. Bone seeking radiopharmaceuticals for palliation of pain in cancer patients with osseous metastases. Anticancer Agents Med Chem 2007;7:381–97. doi:10.2174/187152007781058596.
Ricci S, Boni G, Pastina I, Genovesi D, Cianci C, Chiacchio S, et al. Clinical benefit of bone-targeted radiometabolic therapy with 153Sm-EDTMP combined with chemotherapy in patients with metastatic hormone-refractory prostate cancer. Eur J Nucl Med Mol Imaging 2007;34:1023–30. doi:10.1007/s00259-006-0343-8.
Sciuto R, Festa A, Rea S, Pasqualoni R, Bergomi S, Petrilli G, et al. Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J Nucl Med 2002;43:79–86.
Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;357:336–41. doi:10.1016/S0140-6736(00)03639-4.
Lam MG, Zonnenberg BA. Multimodality treatment in hormone-refractory prostate cancer patients with bone metastases. Eur J Nucl Med Mol Imaging 2008;35:1394–5. doi:10.1007/s00259-008-0782-5.
Lin WY, Lin CP, Yeh SJ, Hsieh BT, Tsai ZT, Ting G, et al. Rhenium-188 hydroxyethylidene diphosphonate: a new generator-produced radiotherapeutic drug of potential value for the treatment of bone metastases. Eur J Nucl Med 1997;24:590–5.
Maxon HR III, Schroder LE, Washburn LC, Thomas SR, Samaratunga RC, Biniakiewicz D, et al. Rhenium-188(Sn)HEDP for treatment of osseous metastases. J Nucl Med 1998;39:659–63.
Li S, Liu J, Zhang H, Tian M, Wang J, Zheng X. Rhenium-188 HEDP to treat painful bone metastases. Clin Nucl Med 2001;26:919–22. doi:10.1097/00003072-200111000-00006.
Liepe K, Kropp J, Runge R, Kotzerke J. Therapeutic efficiency of rhenium-188-HEDP in human prostate cancer skeletal metastases. Br J Cancer 2003;89:625–9. doi:10.1038/sj.bjc.6601158.
Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: tandomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol 2003;21:2869–75. doi:10.1200/JCO.2003.12.060.
Zhang H, Tian M, Li S, Liu J, Tanada S, Endo K. Rhenium-188-HEDP therapy for the palliation of pain due to osseous metastases in lung cancer patients. Cancer Biother Radiopharm 2003;18:719–26. doi:10.1089/108497803770418265.
Wagstaff AJ, Ibbotson T, Goa KL. Capecitabine: a review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer. Drugs 2003;63:217–36. doi:10.2165/00003495-200363020-00009.
Walko CM, Lindley C. Capecitabine: a review. Clin Ther 2005;27:23–44. doi:10.1016/j.clinthera.2005.01.005.
Patel A, Puthillath A, Yang G, Fakih MG. Neoadjuvant chemoradiation for rectal cancer: is more better? Oncology (Williston Park) 2008;22:814–26.
Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975–2005). J Clin Oncol 2008;26:3511–6. doi:10.1200/JCO.2007.15.8782.
Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 2004;22:2214–32. doi:10.1200/JCO.2004.08.009.
Touffet S, Gayet G, Samperez S, Jouan P. Demonstration of thymidine phosphorylase activity in human healthy, adenomatous and cancerous prostate (in French). Bull Cancer 1992;79:151–9.
Sivridis E, Giatromanolaki A, Papadopoulos I, Gatter KC, Harris AL, Koukourakis MI. Thymidine phosphorylase expression in normal, hyperplastic and neoplastic prostates: correlation with tumour associated macrophages, infiltrating lymphocytes, and angiogenesis. Br J Cancer 2002;86:1465–71. doi:10.1038/sj.bjc.6600281.
Ben-Josef E. Capecitabine and radiotherapy as neoadjuvant treatment for rectal cancer. Am J Clin Oncol 2007;30:649–55. doi:10.1097/COC.0b013e3180ca7c9e.
Blake GM, Zivanovic MA, McEwan AJ, Ackery DM. Sr-89 therapy: strontium kinetics in disseminated carcinoma of the prostate. Eur J Nucl Med 1986;12:447–54.
Palmedo H, Guhlke S, Bender H, Sartor J, Schoeneich G, Risse J, et al. Dose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastases. Eur J Nucl Med 2000;27:123–30. doi:10.1007/s002590050017.
Liepe K, Hliscs R, Kropp J, Runge R, Knapp FF Jr, Franke WG. Dosimetry of 188Re-hydroxyethylidene diphosphonate in human prostate cancer skeletal metastases. J Nucl Med 2003;44:953–60.
Ishitsuka H. Capecitabine: preclinical pharmacology studies. Invest New Drugs 2000;18:343–54. doi:10.1023/A:1006497231579.
El Rayes BF, Black CA, Ensley JF. Hormone-refractory prostate cancer responding to capecitabine. Urology 2003;61:462. doi:10.1016/S0090-4295(02)02248-3.
Morant R, Bernhard J, Dietrich D, Gillessen S, Bonomo M, Borner M, et al. Capecitabine in hormone-resistant metastatic prostatic carcinoma—a phase II trial. Br J Cancer 2004;90:1312–7. doi:10.1038/sj.bjc.6601673.
Spicer J, Plunkett T, Somaiah N, Chan S, Kendall A, Bolunwu N, et al. Phase II study of oral capecitabine in patients with hormone-refractory prostate cancer. Prostate Cancer Prostatic Dis 2005;8:364–8. doi:10.1038/sj.pcan.4500821.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12. doi:10.1056/NEJMoa040720.
Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res 1998;4:1013–9.
Fischel JL, Ferrero JM, Formento P, Ciccolini J, Renee N, Formento JL, et al. Taxotere-5′-deoxy-5-fluorouridine combination on hormone-refractory human prostate cancer cells. Anticancer Drugs 2005;16:309–16. doi:10.1097/00001813-200503000-00010.
Ferrero JM, Chamorey E, Oudard S, Dides S, Lesbats G, Cavaglione G, et al. Phase II trial evaluating a docetaxel-capecitabine combination as treatment for hormone-refractory prostate cancer. Cancer 2006;107:738–45. doi:10.1002/cncr.22070.
Kolodziej M, Neubauer MA, Rousey SR, Pluenneke RE, Perrine G, Mull S, et al. Phase II trial of docetaxel/capecitabine in hormone-refractory prostate cancer. Clin Genitourin Cancer 2006;5:155–61. doi:10.3816/CGC.2006.n.033.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lam, M.G.E.H., Bosma, T.B., van Rijk, P.P. et al. 188Re-HEDP combined with capecitabine in hormone-refractory prostate cancer patients with bone metastases: a phase I safety and toxicity study. Eur J Nucl Med Mol Imaging 36, 1425–1433 (2009). https://doi.org/10.1007/s00259-009-1119-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1119-8