Abstract
Background
Rhenium-188-HEDP is a beta-emitting radiopharmaceutical used for palliation of metastatic bone pain. We investigated whether the addition of rhenium-188-HEDP to docetaxel/prednisone improved efficacy of chemotherapy in patients with CRPC.
Methods
Patients with progressive CRPC and osteoblastic bone metastases were randomised for first-line docetaxel 75 mg/m2 3-weekly plus prednisone with or without 2 injections of rhenium-188-HEDP after the third (40 MBq/kg) and after the sixth (20 MBq/kg) cycle of docetaxel. Primary endpoint was progression-free survival (PFS), defined as either PSA, radiographic or clinical progression. Patients were stratified by extent of bone metastases and hospital.
Results
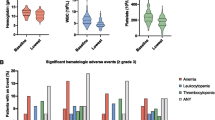
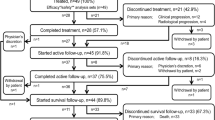
Forty-two patients were randomised for standard treatment and 46 patients for combination therapy. Median number of cycles of docetaxel was 9 in the control group and 8 in the experimental group. Median follow-up was 18.4 months. Two patients from the experimental group did not start treatment after randomisation. In the intention to treat analysis no differences in PFS, survival and PSA became apparent between the two groups. In an exploratory per-protocol analysis median overall survival was significantly longer in the experimental group (33.8 months (95%CI 31.75–35.85)) than in the control group (21.0 months (95%CI 13.61–28.39); p 0.012). Also median PFS in patients with a baseline phosphatase >220U/L was significantly better with combination treatment (9.0 months (95%CI 3.92–14.08) versus 6.2 months (95%CI 3.08–9.32); log rank p 0.005). As expected, thrombocytopenia (grade I/II) was reported more frequently in the experimental group (25% versus 0%).
Conclusion
Combined treatment with rhenium-188-HEDP and docetaxel did not prolong PFS in patients with CRPC. The observed survival benefit in the per-protocol analysis warrants further studies in the combined treatment of chemotherapy and radiopharmaceuticals.


Similar content being viewed by others
References
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20.
Van Dodewaard-de Jong JM, Verheul HMW, Bloemendal HJ, et al. New treatment options for patients with metastatic prostate cancer: what is the optimal sequence? Clin Genitourin Cancer. 2015;13:271–9.
Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387:70–82.
Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213–23.
Van Dodewaard-de Jong JM, Oprea-Lager DE, Hooft L, et al. Radiopharmaceuticals for palliation of bone pain in patients with castration-resistant prostate cancer metastatic to bone: a systematic review. Eur Urol. 2015;70:416–26.
D’Angelo G, Sciuto R, Salvatori M, et al. Targeted “bone-seeking” radiopharmaceuticals for palliative treatment of bone metastases: a systematic review and meta-analysis. Q J Nucl Med Mol Imaging. 2012;56:538–43.
Palmedo H, Manka-Waluch A, Albers P, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new high-energy radiopharmaceutical rhenium-188-hydroxyethylidenediphosphonate. J Clin Oncol. 2003;21:2869–75.
Tu SM, Millikan RE, Mengistu B, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–41.
Van Dodewaard-de Jong JM, de Klerk JMH, Bloemendal HJ, et al. A phase I study of combined docetaxel and repeated high activity 186Re-HEDP in castration-resistant prostate cancer (CRPC) metastatic to bone (the TAXIUM trial). Eur J Nucl Med Mol Imaging. 2011;38:1990–8.
Blake GM, Zivanovic MA, McEwan AJ, Ackery DM. Sr-89 therapy: strontium kinetics in disseminated carcinoma of the prostate. Eur J Nucl Med. 1986;12:447–54.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Petrylak DP, Vogelzang NJ, Budnik N, et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): a randomised double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2015;16:417–25.
Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY); a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–16.
Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol. 2013;14:893–900.
Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol. 2013;14:760–8.
Fizazi K, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–7.
Meulenbeld HJ, van Werkhoven ED, Coenen JL, et al. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic Castration Resistant Prostate Cancer (CRPC), the Netherlands Prostate Study (NePro). Eur J Cancer. 2012;48:2993–3000.
Ter Heine R, Lange R, Breukels O, et al. Bench to bedside development of GMP grade Rhenium-188-HEDP, a radiopharmaceutical for targeted treatment of painful bone metastases. Int J Pharm. 2014;465:317–24.
Dworkin RH, Turk DC, Peirce-Sandner S, et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012;153:1148–58.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by KWF Kankerbestrijding with a subsidy for data management.
Conflict of interest
Mrs van Dodewaard-de Jong states that she has no conflict of interest. Dr. de Klerk states that he has no conflict of interest. Dr. Bloemendal states that he has no conflict of interest. Dr. Oprea-Lager states that she has no conflict of interests. Prof Hoekstra states that he has no conflict of interest. Mr. van den Berg has been paid by Janssen, Astellas, Merck and Bayer for a consulting and/or advisory role. Dr. Los states that she has no conflict of interest. Mr. Beeker states that he has no conflict of interest. Dr. Jonker states that she has no conflict of interest. Professor O’Sullivan has received honoraria from Astellas, Bayer and Janssen. In addition he has received research funding from Bayer. Professor Verheul received honoraria (paid to the institution and not to him personally) from Boehringer Ingelheim for an consulting and advisory role. In addition he has received research funding from Amgen, VHS, Immunovo BV, Sanofi Genzyme and Roche. Dr. van den Eertwegh received honoraria from Astellas and Bayer. In addition he has received research funding from Sanofi Genzyme.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
van Dodewaard-de Jong, J.M., de Klerk, J.M.H., Bloemendal, H.J. et al. A randomised, phase II study of repeated rhenium-188-HEDP combined with docetaxel and prednisone versus docetaxel and prednisone alone in castration-resistant prostate cancer (CRPC) metastatic to bone; the Taxium II trial. Eur J Nucl Med Mol Imaging 44, 1319–1327 (2017). https://doi.org/10.1007/s00259-017-3673-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3673-9