Abstract
Introduction
Alzheimer’s disease (AD) is a very complex neurodegenerative disorder, the exact cause of which is still not known. The major histopathological features, amyloid plaques and neurofibrillary tangles, already described by Alois Alzheimer, have been the focus in research for decades. Despite a probable whole cascade of events in the brain leading to impairment of cognition, amyloid is still the target for diagnosis and treatment.
Discussion
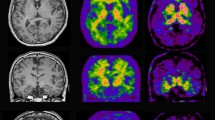
The rapid development of molecular imaging techniques now allows imaging of amyloid plaques in vivo in Alzheimer patients by PET amyloid ligands such as Pittsburgh compound B (PIB). Studies so far have revealed high 11C-PIB retention in brain at prodromal stages of AD and a possibility to discriminate AD from other dementia disorders by 11C-PIB. Ongoing studies are focussing to understand the relationship between brain and CSF amyloid processes and cognitive processes.
Conclusion
In vivo imaging of amyloid will be important for early diagnosis and evaluation of new anti-amyloid therapies in AD.


Similar content being viewed by others
References
Wimo A, Winblad B, Jönsson L. An estimate of the total worldwide societal cost of dementia 2005. Alzheimer & Dementia. 2007;3:81–91.
Brookmeyer R, Johnson E, Ziegler-Graha K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer & Dementia. 2007;3:186–91.
Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–7.
Thal DR, Rub U, Orantoes M, Braak H. Phases of A-beta deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800.
Hardy JA, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6.
Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–9.
Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004;3:519–27.
Shoghi-Jadid K, Small D, Agdeppa ED, Kepe V, Ercoli LM, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brain of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2004;10:24–35.
Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19.
Verhoeff NP, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, et al. In vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–95.
Kudo Y, Okamura N, Furumoto S, et al. 2-(2-[2-Dimethylaminothiazol-5-yl] Ethenyl)-6-(2-[Fluoro]Ethoxy)Benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J Nucl Med. 2006;48:553–61.
Agdeppa ED, Kepe V, Liu J, et al. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphtylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer’s disease. J Neurosci. 2001;21:1–5.
Agdeppa ED, Kepe V, Liu J, et al. Dialkylamino-6-acylmalonnonitrile substituted naphthalenes (DDNP analogs): novel diagnostic and therapeutic tool in Alzheimer’s disease. Mol Imaging Biol. 2003;5:404–17.
Ye L, Morgenstern JL, Gee AD. Delineation of PET imaging agent binding sites on beta-amyloid peptide fibrils. J Biol Chem. 2005;280:23599–60.
Klunk WE, Lopresti BJ, Ikonomovic MD, et al. Binding of positron emission tomography tracer Pittsburgh Compound-B reflects the amount of amyloid-β in Alzheimer’s disease brain but not in transgenic mice brain. J Neurosci. 2005;25:10598–606.
Kung M-P, Hou C, Zhuang Z-P, Skovronsky D, Kung HF. Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissue of patients with Alzheimer’s disease. Brain Res. 2004;1025:98–105.
Toyama H, Ye D, Ichise M, et al. PET imaging of brain with the β-amyloid probe, [11C]6-OH-BTA-1, in a transgenic mouse model of Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2005;32:593–600.
Maeda J, Bib J, Irie T, et al. Longitudinal, quantitative assessment of amyloid, neuroinflammation, and anti-amyloid treatment in a living mouse model of Alzheimer’s disease enable by positron emission tomography. J Neurosci. 2007;10:10957–68.
Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modelling of amyloid binding in humans using PET imaging and Pittsburgh compound-B. J Cereb Blood Flow Metab. 2005;25:1528–47.
Archer HA, Edison P, Brooks DJ, et al. Amyloid load and cerebral atrophy in Alzheimer’s disease: an 11C-PIB positron emission tomography study. Ann Neurol. 2006;60:145–7.
Kemppainen NM, Aalto S, Wilson IA, et al. Voxel-based analysis of PET amyloid ligand [11C]PIB uptake in Alzheimer disease. Neurology. 2006;67:1575–80.
Mintun MA, LaRosso GN, Dence CS, et al. [11C] PIB in a nondemented population. Potentional antecendent marker of Alzheimer’s disease. Neurology. 2006;67:446–52.
Rowe CC, Ng S, Ackermann U, et al. Imaging β-amyloid burden in aging and dementia. Neurology. 2007;68:1718–25.
Engler H, Forsberg A, Almkvist O, et al. Two year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–66.
Kempainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–6.
Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2007 May 10 (in press).
Pike KE, Savage G, Villemagne VL, et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain. 2007;130:2037–844.
Small G, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med 2006;355:2652–63.
Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45:358–68.
Kemppainen NM, Aalto S, Karrasch M, et al. Cognitive reserve hypothesis: Pittsburgh compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2007 Nov 19 (in press).
Josephs KA, Whitwell JL, Ahmed Z, et al. β-amyloid burden is not associated with rates of atrophy. Ann Neurol. 2007 Sept 25 (in press).
Fagan AM, Roe CM, Xiong C, et al. Cerebrospinal fluid tau/β-amyloid 42 ratio as a predictor of cognitive decline in nondemented older adults. Neurology 2007;64:343–9.
Rabinovici GD, Furst AJ, O’Neil, JP, et al. 11C-PIB PET imaging in Alzheimer disease and frontotemporal lobar degeneration. Neurology 2007;68:1205–12.
Engler H, Fritzell Santillo A, Wang SX, et al. In vivo amyloid imaging with PET in frontotemporal dementia. Eur J Nucl Mol Imaging. 2008;35:100–6.
Drzezga A, Grimmer T, Henriksen G, et al. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer’s disease. Neuroimage. 2008;39:619–33.
Johansson A, Savitcheva I, Forsberg A, et al. [11C]-PIB imaging in patients with Parkinson’s disease: preliminary results. Parkinsonism Relat Disord. 2007 Sept 11 (in press).
Maetzler W, Reimold M, Liepelt I, et al. [11]PIB binding in Parkinson’s disease dementia. Neuroimage. 2007 Oct 22 (in press).
Fodero MT, Smith DP, McLean CA, et al. In vitro characterization of Pittsburgh compound-B binding to Lewy bodies. J Neurosci 2007;27:10365–71.
Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–34.
Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553–62.
Fox NC, Black RS, Gilman S, et al. Effect of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005;64:1563–72.
Nicoll JA, Wilkinson D, Holmes C, et al. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med 2003;9:448–52.
Masliah E, Hansen L, Adame A, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 2005;64:129–31.
Mathis CA, Lopresti BJ, Klunk WE. Impact of amyloid imaging on drug development in Alzheimer’s disease. Nucl Med Biol 2007;34:809–22.
Kadir A, Andreasen N, Almkvist O, et al. Effect of phenserine treatment on brain functional activity and amyloid in AD. Ann Neurol 2008 (in press).
Acknowledgement
The financial support of the Swedish Research Council (project no 05817), Stohnes foundation, Foundation of Old Servants, KI foundations, The Alzheimer foundation in Sweden, Swedish Brain Power, and the EC-FP5-project NCI-MCI, QLK6-CT-2000-00502 is gratefully acknowledged.
Conflict of interest statement
There are no conflicts of interest for the author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nordberg, A. Amyloid plaque imaging in vivo: current achievement and future prospects. Eur J Nucl Med Mol Imaging 35 (Suppl 1), 46–50 (2008). https://doi.org/10.1007/s00259-007-0700-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0700-2