Abstract
Purpose
The association of the regional cerebral metabolic rate of glucose utilisation (rCMRglc) and years of schooling has been extensively studied in Alzheimer’s disease (AD). The results suggest that brain reserve capacity (BRC) allows patients with more years of schooling to cope better with AD pathology. The objective of this study was to provide initial evidence for BRC in frontotemporal dementia (FTD).
Methods
Twenty-nine patients with FTD and 16 healthy age- and education-matched controls underwent PET imaging of the brain with 18F-fluoro-2-deoxy-glucose. A group comparison of rCMRglc was conducted between patients and controls and the output was saved as region of interest (ROI). A linear regression analysis with education as the independent and rCMRglc as the dependent variable, adjusted for age, gender and total score on the CERAD neuropsychological battery, was conducted in SPM2 over the pre-assigned ROI.
Results
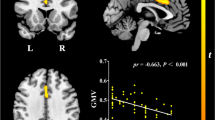
Patients showed a reduced rCMRglc in almost the entire prefrontal cortex and the anterior cingulate cortex as compared with controls (p < 0.05 corrected for multiple comparisons). The regression analysis revealed a significant negative association between years of schooling and rCMRglc in the bilateral inferior frontal cortex (p < 0.001, uncorrected for multiple comparisons), which was independent of demographic variables and cognitive performance level. There was a strong negative correlation of rCMRglc and education (r = −0.45).
Conclusion
The study provides initial evidence for BRC in FTD. The findings suggest that interindividual differences in educational level affect BRC by partially mediating the relationship between neurodegeneration and the clinical manifestation of FTD.


Similar content being viewed by others
References
Katzman R. Education and the prevalence of dementia and Alzheimer’s disease. Neurology 1993;43:13–20.
Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994;271:1004–10.
Bennett DA, Wilson RS, Schneider JA, Evans DA, Mendes de Leon CF, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology 2003;60:1909–15.
Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 1992;32:371–5.
Perneczky R, Drzezga A, Diehl-Schmid J, Schmid G, Wohlschlager A, Kars S, et al. Schooling mediates brain reserve in Alzheimer’s disease: findings of FDG PET. J Neurol Neurosurg Psychiatry 2006;77:1060–3.
Scarmeas N, Zarahn E, Anderson KE, Habeck CG, Hilton J, Flynn J, et al. Association of life activities with cerebral blood flow in Alzheimer disease: implications for the cognitive reserve hypothesis. Arch Neurol 2003;60:359–65.
Sundström T. Human brain function evaluated with rCBF-SPECT. Umeo: Faculty of Medicine, Radiation Sciences 2006;78.
Cao Q, Jiang K, Zhang M, Liu Y, Xiao S, Zuo C, et al. Brain glucose metabolism and neuropsychological test in patients with mild cognitive impairment. Chin Med J 2003;116:1235–8.
Glatt SL, Hubble JP, Lyons K, Paolo A, Troster AI, Hassanein RE, et al. Risk factors for dementia in Parkinson’s disease: effect of education. Neuroepidemiology 1996;15:20–5.
Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology 2003;61:349–54.
Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry 1994;57:416–8.
Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull 1988;24:641–52.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98.
Chandler MJ, Lacritz LH, Hynan LS, Barnard HD, Allen G, Deschner M, et al. A total score for the CERAD neuropsychological battery. Neurology 2005;65:102–6.
Edwards-Lee T, Miller BL, Benson DF, Cummings JL, Russell GL, Boone K, et al. The temporal variant of frontotemporal dementia. Brain 1997;120 Pt 6:1027–40.
Drzezga A, Riemenschneider M, Strassner B, Grimmer T, Peller M, Knoll A, et al. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology 2005;64:102–7.
Talairach J, Tournoux P. Co-planar stereotactical atlas of the human brain: 3-dimensional proportional system—an approach to cerebral imaging. New York: Thieme Medical; 1988.
Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, Kurz A. Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry 2006;14:139–44.
Diehl J, Grimmer T, Drzezga A, Riemenschneider M, Forstl H, Kurz A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol Aging 2004;25:1051–6.
Dennis M, Spiegler BJ, Hetherington R. New survivors for the new millennium: cognitive risk and reserve in adults with childhood brain insults. Brain Cogn 2000;42:102–5.
Diehl-Schmid J, Grimmer T, Drzezga A, Bornschein S, Riemenschneider M, Forstl H, et al. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol Aging 2007;28:42–50.
Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol 2005;62:925–30.
Acknowledgements
This study was partly funded by the Federal Ministry of Research and Education as part of a national collaboration on dementia (Kompetenznetz Demenzen), grant N° 01GI0420 and the Komission für Klinische Forschung, Klinikum rechts der Isar, München, grant N° 8765. The sponsors played no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review and approval of the manuscript. The authors do not report any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perneczky, R., Diehl-Schmid, J., Drzezga, A. et al. Brain reserve capacity in frontotemporal dementia: a voxel-based 18F-FDG PET study. Eur J Nucl Med Mol Imaging 34, 1082–1087 (2007). https://doi.org/10.1007/s00259-006-0323-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0323-z