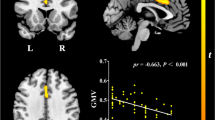
Abstract
Objective
Formal education and other cognitive challenges influence brain structure and improve function. It is believed that cognitive activities create a cognitive reserve (CR) that can slow the decline due to aging and neurodegenerative diseases. This study investigated alterations of regional cerebral blood flow (rCBF) associated with high and low CR in different stages of Alzheimer’s disease (AD) and examined whether rCBF alteration mediates the relationship between education and cognitive performance.
Methods
Patients with AD or amnestic mild cognitive impairment (aMCI) and healthy controls were divided into low cognitive reserve (LCR) and high cognitive reserve (HCR) subgroups according to median of education years (≤ 9 vs. > 9 years). The final study population included 89 AD patients (67 LCR, 22 HCR), 74 aMCI patients (44 LCR, 30 HCR), and 66 healthy controls (29 LCR, 37 HCR). All subjects were examined by arterial spin labeling magnetic resonance imaging and a neurocognitive test battery. rCBF was compared among groups by two-way analysis of variance. Mediation analyses were used to explore the relationships among education, rCBF, and cognitive test scores.
Results
There were significant interaction effects of disease state (AD, aMCI, HC) and education level (LCR, HCR) on CBF in right hippocampus, posterior cingulate cortex, and right inferior parietal cortex (R_IPC). Education regulated episodic memory score by influencing right hippocampal CBF in HC_HCR and aMCI_HCR subgroups.
Conclusion
Our results indicate that the protective effect of education against cognitive dysfunction in early-stage AD is mediated at least partially by altered CBF in right hippocampus.





Similar content being viewed by others
References
Stern Y (2012) Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol 11(11):1006–1012. https://doi.org/10.1016/S1474-4422(12)70191-6
Stern Y, Barnes CA, Grady C, Jones RN, Raz N (2019) Brain reserve, cognitive reserve, compensation, and maintenance: operationalization, validity, and mechanisms of cognitive resilience. Neurobiol Aging 83:124–129. https://doi.org/10.1016/j.neurobiolaging.2019.03.022
Stern Y (2009) Cognitive reserve. Neuropsychologia 47(10):2015–2028. https://doi.org/10.1016/j.neuropsychologia.2009.03.004
Perneczky R, Drzezga A, Diehl-Schmid J, Schmid G, Wohlschlager A, Kars S et al (2006) Schooling mediates brain reserve in Alzheimer's disease: findings of fluoro-deoxy-glucose-positron emission tomography. J Neurol Neurosurg Psychiatry 77(9):1060–1063. https://doi.org/10.1136/jnnp.2006.094714
Querbes O, Aubry F, Pariente J, Lotterie JA, Demonet JF, Duret V et al (2009) Early diagnosis of Alzheimer's disease using cortical thickness: impact of cognitive reserve. Brain 132(Pt 8):2036–2047. https://doi.org/10.1093/brain/awp105
Adam S, Bonsang E, Grotz C, Perelman S (2013) Occupational activity and cognitive reserve: implications in terms of prevention of cognitive aging and Alzheimer's disease. Clin Interv Aging 8:377–390. https://doi.org/10.2147/CIA.S39921
Wang J, Peng G, Liu P, Tan X, Luo B (2019) Regulating effect of CBF on memory in cognitively normal older adults with different ApoE genotype: the Alzheimer's Disease Neuroimaging Initiative (ADNI). Cogn Neurodyn 13(6):513–518. https://doi.org/10.1007/s11571-019-09536-x
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci U S A 98(2):676–682. https://doi.org/10.1073/pnas.98.2.676
Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME (2010) Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A 107(41):17757–17762. https://doi.org/10.1073/pnas.1010459107
Takahashi M, Tada T, Nakamura T, Koyama K, Momose T (2019) Efficacy and Limitations of rCBF-SPECT in the Diagnosis of Alzheimer's Disease With Amyloid-PET. Am J Alzheimers Dis Other Demen 34(5):314–321. https://doi.org/10.1177/1533317519841192
Yoshii F, Kawaguchi C, Kohara S, Shimizu M, Onaka H, Ryo M et al (2018) Characteristic deterioration of ADAS-Jcog subscale scores and correlations with regional cerebral blood flow reductions in Alzheimer's disease. Neurol Sci 39(5):909–918. https://doi.org/10.1007/s10072-018-3277-6
Marchitelli R, Aiello M, Cachia A, Quarantelli M, Cavaliere C, Postiglione A et al (2018) Simultaneous resting-state FDG-PET/fMRI in Alzheimer Disease: Relationship between glucose metabolism and intrinsic activity. Neuroimage 176:246–258. https://doi.org/10.1016/j.neuroimage.2018.04.048
Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X (2016) Arterial Spin Labeling Perfusion of the Brain: Emerging Clinical Applications. Radiology 281(2):337–356. https://doi.org/10.1148/radiol.2016150789
Wolk DA, Detre JA (2012) Arterial spin labeling MRI: an emerging biomarker for Alzheimer's disease and other neurodegenerative conditions. Curr Opin Neurol 25(4):421–428. https://doi.org/10.1097/WCO.0b013e328354ff0a
Musiek ES, Chen Y, Korczykowski M, Saboury B, Martinez PM, Reddin JS et al (2012) Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer's disease. Alzheimers Dement 8(1):51–59. https://doi.org/10.1016/j.jalz.2011.06.003
Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM (2009) Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology 250(3):856–866. https://doi.org/10.1148/radiol.2503080751
Duan W, Zhou GD, Balachandrasekaran A, Bhumkar AB, Boraste PB, Becker JT et al (2021) Cerebral Blood Flow Predicts Conversion of Mild Cognitive Impairment into Alzheimer's Disease and Cognitive Decline: An Arterial Spin Labeling Follow-up Study. J Alzheimers Dis 82(1):293–305. https://doi.org/10.3233/JAD-210199
Zlatar ZZ, Wierenga CE, Bangen KJ, Liu TT, Jak AJ (2014) Increased hippocampal blood flow in sedentary older adults at genetic risk for Alzheimer's disease. J Alzheimers Dis 41(3):809–817. https://doi.org/10.3233/JAD-132252
Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP et al (2012) Assessment of Alzheimer's disease risk with functional magnetic resonance imaging: an arterial spin labeling study. J Alzheimers Dis 31(Suppl 3):S59–S74. https://doi.org/10.3233/JAD-2012-120292
Grothe MJ, Teipel SJ (2016) Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer's disease correspond to dissociable functional brain networks. Hum Brain Mapp 37(1):35–53. https://doi.org/10.1002/hbm.23018
Zhu J, Zhuo C, Qin W, Xu Y, Xu L, Liu X et al (2015) Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res 63:28–35. https://doi.org/10.1016/j.jpsychires.2015.03.002
Melie-Garcia L, Sanabria-Diaz G, Sanchez-Catasus C (2013) Studying the topological organization of the cerebral blood flow fluctuations in resting state. Neuroimage 64:173–184. https://doi.org/10.1016/j.neuroimage.2012.08.082
Franzmeier N, Caballero MÁA, Taylor ANW, Simon-Vermot L, Buerger K, Ertl-Wagner B, Mueller C, Catak C, Janowitz D, Baykara E, Gesierich B, Duering M, Ewers M, Alzheimer’s Disease Neuroimaging Initiative (2017) Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain imaging and behavior 11(2):368–382. https://doi.org/10.1007/s11682-016-9599-1
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34(7):939–944. https://doi.org/10.1212/wnl.34.7.939
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH et al (2011) The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7(3):263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7(3):270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ et al (2010) Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74(3):201–209. https://doi.org/10.1212/WNL.0b013e3181cb3e25
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56(3):303–308. https://doi.org/10.1001/archneur.56.3.303
Xu G, Rowley HA, Wu G, Alsop DC, Shankaranarayanan A, Dowling M et al (2010) Reliability and precision of pseudo-continuous arterial spin labeling perfusion MRI on 3.0 T and comparison with 15O-water PET in elderly subjects at risk for Alzheimer's disease. NMR Biomed 23(3):286–293. https://doi.org/10.1002/nbm.1462
Aslan S, Lu H (2010) On the sensitivity of ASL MRI in detecting regional differences in cerebral blood flow. Magn Reson Imaging 28(7):928–935. https://doi.org/10.1016/j.mri.2010.03.037
Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V et al (2008) Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer's disease. Ann Neurol 63(1):112–118. https://doi.org/10.1002/ana.21212
Mungas D, Gavett B, Fletcher E, Farias ST, DeCarli C, Reed B (2018) Education amplifies brain atrophy effect on cognitive decline: implications for cognitive reserve. Neurobiol Aging 68:142–150. https://doi.org/10.1016/j.neurobiolaging.2018.04.002
Rane S, Ally BA, Hussey E, Wilson T, Thornton-Wells T, Gore JC, Donahue MJ (2013) Inverse correspondence between hippocampal perfusion and verbal memory performance in older adults. Hippocampus 23(3):213–220. https://doi.org/10.1002/hipo.22080
Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 8(3):448–460
Weiler M, Casseb RF, de Campos BM, de Ligo Teixeira CV, Carletti-Cassani AFMK, Vicentini JE, Magalhães TNC, de Almeira DQ, Talib LL, Forlenza OV, Balthazar MLF, Castellano G (2018) Cognitive Reserve Relates to Functional Network Efficiency in Alzheimer's Disease. Front Aging Neurosci 10:255. https://doi.org/10.3389/fnagi.2018.00255
Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, Bondi MW (2012) Assessment of Alzheimer's disease risk with functional magnetic resonance imaging: an arterial spin labeling study. J Alzheimer's Dis 31(Suppl 3):S59–S74. https://doi.org/10.3233/JAD-2012-120292
Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB (2009) Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiology of aging 30(11):1737–1748. https://doi.org/10.1016/j.neurobiolaging.2008.01.012
Sabariego M, Tabrizi NS, Marshall GJ, McLagan AN, Jawad S, Hales JB (2021) In the temporal organization of episodic memory, the hippocampus supports the experience of elapsed time. Hippocampus 31(1):46–55. https://doi.org/10.1002/hipo.23261
Raynal E, Schnider A, Manuel AL (2020) Early signal from the hippocampus for memory encoding. Hippocampus 30(2):114–120. https://doi.org/10.1002/hipo.23137
Raichle ME (2015) The brain's default mode network. Annu Rev Neurosci 38:433–447. https://doi.org/10.1146/annurev-neuro-071013-014030
Palmqvist S, Scholl M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H et al (2017) Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 8(1):1214. https://doi.org/10.1038/s41467-017-01150-x
Buxton RB, Uludag K, Dubowitz DJ, Liu TT (2004) Modeling the hemodynamic response to brain activation. Neuroimage 23(Suppl 1):S220–S233. https://doi.org/10.1016/j.neuroimage.2004.07.013
Zheng W, Cui B, Han Y, Song H, Li K, He Y et al (2019) Disrupted Regional Cerebral Blood Flow, Functional Activity and Connectivity in Alzheimer's Disease: A Combined ASL Perfusion and Resting State fMRI Study. Front Neurosci 13:738. https://doi.org/10.3389/fnins.2019.00738
Wu X, Li R, Fleisher AS, Reiman EM, Guan X, Zhang Y et al (2011) Altered default mode network connectivity in Alzheimer's disease--a resting functional MRI and Bayesian network study. Hum Brain Mapp 32(11):1868–1881. https://doi.org/10.1002/hbm.21153
Fiehler K, Bannert MM, Bischoff M, Blecker C, Stark R, Vaitl D et al (2011) Working memory maintenance of grasp-target information in the human posterior parietal cortex. Neuroimage 54(3):2401–2411. https://doi.org/10.1016/j.neuroimage.2010.09.080
Singh-Curry V, Husain M (2009) The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47(6):1434–1448. https://doi.org/10.1016/j.neuropsychologia.2008.11.033
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Fund number: 81901726, 81771817, 82071905). We would like to thank the many collaborators for their work in setting up the study and implementing the protocol. We would like to thank our patients for their role in the research design.
Author information
Authors and Affiliations
Contributions
Wanqiu Zhu: Data collection, Methodology, Software, Formal analysis, Validation, Writing - original draft. Ziwen Gao: Data collection, Data curation, Visualization, Investigation, Software. Hui Li: Data collection. Ziang Hang: Data collection. Xiaohu Li: Methodology, Software. Haibao Wang: Methodology, Software. Xingqi Wu: Data collection, Data curation, Visualization, Investigation. Yanghua Tian: Data curation, Visualization, Investigation. Shanshan Zhou: Data curation, Visualization, Investigation. Xiaoshu Li: Methodology, Software, Formal analysis, Funding acquisition, Writing - review & editing. Yongqiang Yu: Conceptualization, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Medical Research Ethics Committee of the First Affiliated Hospital of Anhui Medical University, China, according to the Declaration of Helsinki. Informed consent was provided by all subjects.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, W., Gao, Z., Li, H. et al. Education reduces cognitive dysfunction in Alzheimer’s disease by changing regional cerebral perfusion: An in-vivo arterial spin labeling study. Neurol Sci 44, 2349–2361 (2023). https://doi.org/10.1007/s10072-023-06696-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06696-x