Abstract
Purpose
We investigated the potential for improvement in disease control by use of autologous peripheral blood stem cell transplant (PBSCT) to permit administration of high activities of 186Re-hydroxyethylidene diphosphonate (HEDP) in patients with progressive hormone-refractory prostate cancer (HRPC).
Methods
Eligible patients had progressive HRPC metastatic to bone, good performance status and minimal soft tissue disease. Patients received 5,000 MBq of 186Re-HEDP i.v., followed 14 days later by PBSCT. Response was assessed using PSA, survival, pain scores and quality of life.
Results
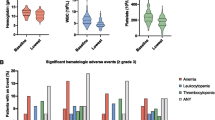
Thirty-eight patients with a median age of 67 years (range 50–77) and a median PSA of 57 ng/ml (range 4–3,628) received a median activity of 4,978 MBq 186Re-HEDP (range 4,770–5,100 MBq). The most serious toxicity was short-lived grade 3 thrombocytopenia in 8 (21%) patients. The median survival of the group is 21 months (95%CI 18–24 months) with Kaplan-Meier estimated 1- and 2-year survival rates of 83% and 40% respectively. Thirty-one patients (81%, 95% CI 66–90%) had stable or reduced PSA levels 3 months post therapy while 11 (29%, 95% CI 15–49%) had PSA reductions of >50% lasting >4 weeks. Quality of life measures were stable or improved in 27 (66%) at 3 months.
Conclusion
We have shown that it is feasible and safe to deliver high-activity radioisotope therapy with PBSCT to men with metastatic HRPC. Response rates and survival data are encouraging; however, further research is needed to define optimal role of this treatment approach.



Similar content being viewed by others
References
Clarke NW. The management of hormone-relapsed prostate cancer. BJU Int 2003;92(8):860–868
Dearnaley DP. Cancer of the prostate. BMJ 1994;308(6931):780–784
Pisters LL. The challenge of locally advanced prostate cancer. Semin Oncol 1999;26(2):202–216
Tannock IF, Osoba D, Stockler MR, Ernst DS, Neville AJ, Moore MJ, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol 1996;14(6):1756–1764
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351(15):1513–1520
Sridhara R, Eisenberger MA, Sinibaldi VJ, Reyno LM, Egorin MJ. Evaluation of prostate-specific antigen as a surrogate marker for response of hormone-refractory prostate cancer to suramin therapy. J Clin Oncol 1995;13(12):2944–2953
Fossa SD, Slee PH, Brausi M, Horenblas S, Hall RR, Hetherington JW, et al. Flutamide versus prednisone in patients with prostate cancer symptomatically progressing after androgen-ablative therapy: a phase III study of the European organization for research and treatment of cancer genitourinary group. J Clin Oncol 2001;19(1):62–71
Kelly WK, Scher HI, Mazumdar M, Vlamis V, Schwartz M, Fossa SD. Prostate-specific antigen as a measure of disease outcome in metastatic hormone-refractory prostate cancer. J Clin Oncol 1993;11(4):607–615
Small EJ, Vogelzang NJ. Second-line hormonal therapy for advanced prostate cancer: a shifting paradigm. J Clin Oncol 1997;15(1):382–388
Lewington VJ, McEwan AJ, Ackery DM, Bayly RJ, Keeling DH, Macleod PM, et al. A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer 1991;27(8):954–958
Porter AT, McEwan AJ, Powe JE, Reid R, McGowan DG, Lukka H, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993;25(5):805–813
Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994;31(1):33–40
Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol 1998;16(4):1574–1581
Han SH, de Klerk JM, Tan S, van het Schip AD, Derksen BH, van Dijk A, et al. The PLACORHEN study: a double-blind, placebo-controlled, randomized radionuclide study with 186Re-etidronate in hormone-resistant prostate cancer patients with painful bone metastases. Placebo Controlled Rhenium Study. J Nucl Med 2002;43(9):1150–1156
Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001;357(9253):336–341
Palmedo H, Manka-Waluch A, Albers P, Schmidt-Wolf IG, Reinhardt M, Ezziddin S, et al. Repeated bone-targeted therapy for hormone-refractory prostate carcinoma: randomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonate. J Clin Oncol 2003;21(15):2869–2875
Deutsch E, Libson K, Vanderheyden JL, Ketring AR, Maxon HR. The chemistry of rhenium and technetium as related to the use of isotopes of these elements in therapeutic and diagnostic nuclear medicine. Int J Rad Appl Instrum B 1986;13(4):465–477
Mathieu L, Chevalier P, Galy G, Berger M. Preparation of rhenium-186 labelled EHDP and its possible use in the treatment of osseous neoplasms. Int J Appl Radiat Isot 1979;30(12):725–727
Maxon HR 3rd, Schroder LE, Hertzberg VS, Thomas SR, Englaro EE, Samaratunga R, et al. Rhenium-186(Sn)HEDP for treatment of painful osseous metastases: results of a double-blind crossover comparison with placebo. J Nucl Med 1991;32(10):1877–1881
Maxon HR 3rd, Schroder LE, Thomas SR, Hertzberg VS, Deutsch EA, Scher HI, et al. Re-186(Sn) HEDP for treatment of painful osseous metastases: initial clinical experience in 20 patients with hormone-resistant prostate cancer. Radiology 1990;176(1):155–159
de Klerk JM, van het Schip AD, Zonnenberg BA, van Dijk A, Stokkel MP, Han SH, et al. Evaluation of thrombocytopenia in patients treated with rhenium-186-HEDP: guidelines for individual dosage recommendations. J Nucl Med 1994;35(9):1423–1428
de Klerk JM, van Dijk A, van het Schip AD, Zonnenberg BA, van Rijk PP. Pharmacokinetics of rhenium-186 after administration of rhenium-186-HEDP to patients with bone metastases. J Nucl Med 1992;33(5):646–651
O’Sullivan JM, McCready VR, Flux G, Norman AR, Buffa FM, Chittenden S, et al. High activity rhenium-186 HEDP with autologous peripheral blood stem cell rescue: a phase I study in progressive hormone refractory prostate cancer metastatic to bone. Br J Cancer 2002;86(11):1715–1720
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 1999;17(11):3461–3467
Graham C, Bond SS, Gerkovich MM, Cook MR. Use of the McGill pain questionnaire in the assessment of cancer pain: replicability and consistency. Pain 1980;8(3):377–387
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85(5):365–376
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351(15):1502–1512
Shah RB, Mehra R, Chinnaiyan AM, Shen R, Ghosh D, Zhou M, et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res 2004;64(24):9209–9216
Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994;31(1):33–40
Buffa FM, Flux GD, Guy MJ, O'Sullivan JM, McCready VR, Chittenden SJ, et al. A model-based method for the prediction of whole-body absorbed dose and bone marrow toxicity for 186Re-HEDP treatment of skeletal metastases from prostate cancer. Eur J Nucl Med Mol Imaging 2003;30(8):1114–1124
Yarnold JR, Horwich A, Duchesne G, Westbrook K, Gibbs JE, Peckham MJ. Chemotherapy and radiotherapy for advanced testicular non-seminoma. I. The influence of sequence and timing of drugs and radiation on the appearance of normal tissue damage. Radiother Oncol 1983;1(2):91–99
Looney WB. Alternating chemotherapy and radiotherapy. NCI Monogr 1988;6(6):85–94
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 2002;94(19):1458–1468
Dearnaley DP, Sydes MR, Mason MD, Stott M, Powell CS, Robinson AC, et al. A double-blind, placebo-controlled, randomized trial of oral sodium clodronate for metastatic prostate cancer (MRC PR05 Trial). J Natl Cancer Inst 2003;95(17):1300–1311
Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, et al. Effect of endothelin—a receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol 2003;21(4):679–689
Acknowledgements
This work was undertaken in The Royal Marsden NHS Trust, who received a proportion of its funding from the NHS Executive; the views expressed in this publication are those of the authors and not necessarily those of the NHS Executive. This work was supported by the Institute of Cancer Research, the Bob Champion Cancer Trust and Cancer Research UK Section of Radiotherapy.
This project was funded by a grant from the National Cancer Institute supported by NIH grant number CA86784-02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Sullivan, J.M., Norman, A.R., McCready, V.R. et al. A phase 2 study of high-activity 186Re-HEDP with autologous peripheral blood stem cell transplant in progressive hormone-refractory prostate cancer metastatic to bone. Eur J Nucl Med Mol Imaging 33, 1055–1061 (2006). https://doi.org/10.1007/s00259-005-0010-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0010-5