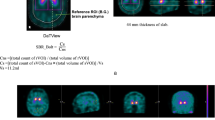
Abstract
Nigral dopaminergic projections to the striatum are targeted in Parkinson’s disease (PD). The extent of the degeneration of the dopaminergic system in PD can be visualised by dopamine transporter imaging using single-photon emission tomography (SPET). In this study in 188 patients with PD, we analysed the image patterns and compared them with the clinical features in order to verify the usefulness of technetium-99m TRODAT-1 brain SPET in the evaluation of patients with PD. Two independent readers visually assessed SPET slices from three brain axes according to a “fine” visual scale; results were also grouped according to a “rough” visual scale. Results of both visual and semi-quantitative analyses were compared among patients with different stages of PD and healthy controls. There was good agreement between the readers in the interpretation of the image patterns [kappa statistic (κ)=0.85 for the presence of PD; κ=0.88 for the rough scale and 0.81 for the fine scale]. Good concordance was obtained when visual interpretation was used to evaluate the presence of PD (sensitivity =98%, specificity =86%, κ=0.85). Semi-quantitative analyses revealed significant negative correlations between both striatal and putaminal uptake and disease severity as assessed using the Hoehn and Yahr scale (ρ=−0.89 and −0.93 respectively). An apparent decrease in striatal uptake in early PD, hardly discernible from the uptake level in advanced PD, was commonly found in visual analyses. The results suggest that both visual and semi-quantitative analyses of 99mTc-TRODAT-1 SPET images reflect neurodegeneration in PD, and that 99mTc-TRODAT-1 SPET represents an adequate means for evaluation of the status of patients with PD.




Similar content being viewed by others
References
Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999; 56:33–39.
Hughes AJ, Daniel SE, Kilford L, Lee AJ. The accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinicopathological study. J Neurol Neurosurg Psychiatry 1992; 55:181–184.
Agid Y. Parkinson’s disease: pathophysiology. Lancet 1991; 337:1321–1324.
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in striatum of patients with idiopathic Parkinson’s disease N Engl J Med 1988; 876:876–880.
Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J Neurosci 1996; 16:436–447.
Chen YK, Liu LS, Huang WS, et al. The role of dopamine transporter imaging agent [99mTc]TRODAT-1 in hemi-parkinsonism rat brain. Nucl Med Biol 2001; 28:923–928.
Wilson JM, Levey AI, Rajput A, et al. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology 1996; 47:718–726.
Mosley PD, Schneider JS, Acton PD, et al. Binding of99mTc-TRODAT-1 to dopamine transporter in patients with Parkinson’s disease and in healthy volunteers. J Nucl Med 2000; 41:584–589.
Niznik HB, Fogel EF, Fassos FF, Seeman P. The dopamine transporter is absent in parkinsonian putamen and reduced in the caudate nucleus. J Neurochem 1991; 56:192–198.
Brooks DJ. PET studies on the early and differential diagnosis of Parkinson’s disease. Neurology 1993; 43 (Suppl 6):S6–S16.
Booij J, Tissingh G, Winogrodzka A, van Royen EZ. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. Eur J Nucl Med 1999; 26:171–182.
Innis RB, Seibyl JB, Scanley BE, et al. Single photon computed imaging demonstrates loss of striatal dopamine transporters in Parkinson’s disease. Proc Natl Acad Sci U S A 1993; 90:11965–11969.
Seibyl JP, Marek KL, Quinlan D, et al. Decreased single-photon emission tomographic [123I]β-CIT SPECT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 1995; 38:589–598.
Marek KL, Seibyl JP, Zoghbi SS, et al. [123I]β-CIT SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 1996; 46:231–237.
Staffen W, Mair A, Unterrainer J, Trinka E, Bsteh C, Ladurner G. [123I]β-CIT binding and SPET compared with clinical diagnosis in parkinsonism. Nucl Med Commun 2000; 21: 417–424.
Kung MP, Stevenson DA, Plossl K, et al. [99mTc]TRODAT-1: a novel technetium-99m complex as dopamine transporter imaging agent. Eur J Nucl Med 1997; 24:372–380.
Huang WS, Lin SZ, Lin JC, Wey SP, Ting G, Liu RS. Evaluation of early-stage Parkinson’s disease with99mTc-TRODAT-1 imaging. J Nucl Med 2001; 42:1303–1308.
Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol 1992; 32:S125–S127.
Hoehn MW, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology (Minneap) 1967; 17:427–442.
Wey SP, Tsai HY, Jong JS, et al. Formulation of a lyophilized kit for preparation of [99mTc]TRODAT-1 [abstract]. Eur J Nucl Med 1998; 25 (Suppl):1164.
Chou YH, Lu RB, Huang WS, Wey SP, Ding G. Dopamine transporter binding and cognitive function in methamphetamine abuser during detoxification: a SPECT study. J Cereb Blood Flow Metab 2003; 23 (Suppl):8.
Snedecor GW, Cochran WG. Specific indices of interrater reliability. In: Snedecor GW, Cochran WG, eds. Statistical methods, 6th edn. Ames, IO: Iowa State University Press; 1996:200–205.
Fleiss JL. Statistical methods for rates and proportions, 2nd edn. New York: John Wiley; 1981:218.
Parkinson Study Group. A multicenter assessment of dopamine transporter imaging with DOPASCAN/SPECT in parkinsonism. Neurology 2000; 55:1540–1547.
Tatsch K. Can SPECT imaging of dopamine uptake sites replace PET imaging in Parkinson’s disease? Eur J Nucl Med Mol Imaging 2002; 29:711–714.
Løkkegarrd A, Werdelin LM, Friberg L. Clinical impact of diagnostic SPET investigations with a dopamine re-uptake ligand. Eur J Nucl Med Mol Imaging 2002; 29:1623–1629.
Catafau AM. Brain SPECT of dopaminergic neurotransmission: a new tool with proved clinical impact. Nucl Med Commun 2001; 22:1059–1060.
Eising EG, Muler T, Freudenberg L, et al. SPECT imaging with [123I]-β-CIT in Parkinsonism: comparison of SPECT images obtained by a single headed and a three-headed gamma camera. Nucl Med Commun 2001; 22:145–150.
Muller TH, Farahati J, Kuhn W, et al. [123I]β-CIT SPECT visualizes dopamine transporters loss in de novo parkinsonian patients. Eur Neurol 1998; 39:44–48.
Brooks DJ. Functional imaging in relation to parkinsonian syndromes. J Neurol Sci 1993; 115:1–17.
van Dyck CH, Seibyl JP, Malison RT, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 2002; 10:36–43.
King MA, Schwinger RB, Penney BC, Doherty PW, Bianco JA. Digital restoration of indium-111 and iodine-123 SPECT images with optimized Metz filters. J Nucl Med 1986; 27:1327–1336.
Kuikka JT, Bergström, Ahonen A, Länsimies E. The dosimetry of iodine-123 labelled 2β-carbomethoxy-3β-(4-iodophenyl)tropane. Eur J Nucl Med 1994; 21:53–56.
Vilkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine transporters with age in healthy human subjects. Ann Neurol 1994; 36:237–239.
Mozley PD, Acton PD, Barraclough ED, et al. Effects of age on dopamine transporters in healthy humans. J Nucl Med 1999; 40:1812–1817.
Marek K, Innis R, Van Dyck C, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology 2001; 57:2089–2094.
Dressel SHJ, Kung MP, Plössl K, Meegalla SK, Kung HF. Pharmacological effects of dopaminergic drugs on in vivo binding of [99mTc]TRODAT-1 to the central dopamine transporter in rats. Eur J Nucl Med 1998; 25:31–39.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, WS., Lee, MS., Lin, JC. et al. Usefulness of brain 99mTc-TRODAT-1 SPET for the evaluation of Parkinson’s disease. Eur J Nucl Med Mol Imaging 31, 155–161 (2004). https://doi.org/10.1007/s00259-003-1331-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1331-x