Abstract
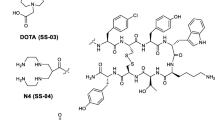
Earlier studies have shown that modification of the octapeptide octreotide in positions 3 and 8 may result in compounds with increased somatostatin receptor affinity that, if radiolabelled, display improved uptake in somatostatin receptor-positive tumours. The aim of a recent research study in our laboratory was to employ the parallel peptide synthesis approach by further exchanging the amino acid in position 3 of octreotide and coupling the macrocyclic chelator DOTA(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) to these peptides for labelling with radiometals like gallium-67 or -68, indium-111, yttrium-90 and lutetium-177. The purpose was to find radiopeptides with an improved somatostatin receptor binding profile in order to extend the spectrum of targeted tumours. A first peptide, [111In,90Y-DOTA]-1-Nal3-octreotide (111In,90Y-DOTA-NOC), was isolated which showed an improved profile. InIII-DOTA-NOC exhibited the following IC50 values (nM) when studied in competition with [125I][Leu8, d-Trp22, Tyr25]somatostatin-28 (values for YIII-DOTA-NOC are shown in parentheses): sstr2, 2.9±0.1 (3.3±0.2); sstr3, 8±2 (26±1.9); sstr5, 11.2±3.5 (10.4±1.6). Affinity towards sstr1 and 4 was very low or absent. InIII-DOTA-NOC is superior to all somatostatin-based radiopeptides having this particular type of binding profile, including DOTA-lanreotide, and has three to four times higher binding affinity to sstr2 than InIII,YIII-DOTA-Tyr3-octreotide (InIII,YIII-DOTA-TOC). In addition, [111In]DOTA-NOC showed a specific and high rate of internalization into AR4-2J rat pancreatic tumour cells which, after 4 h, was about two times higher than that of [111In]DOTA-TOC and three times higher than that of [111In]DOTA-octreotide ([111In]DOTA-OC). The internalized radiopeptides were externalized intact upon 2 h of internalization followed by an acid wash. After 2–3 h of externalization a plateau is reached, indicating a steady-state situation explained by reactivation of the receptors followed by re-endocytosis. Biodistribution studies in CA 20948 tumour-bearing rats showed rapid clearance from all sstr-negative tissues except the kidneys. At 4 h the uptake of [111In]DOTA-NOC in the tumour and sstr-positive tissues, such as adrenals, stomach and pancreas, was three to four times higher than that of [111In]DOTA-TOC. Differential blocking studies indicate that this is at least partially due to the uptake mediated by sstr3 and sstr5. These very promising preclinical data justify the use of this new radiopeptide for imaging and potentially internal radiotherapy studies in patients.





Similar content being viewed by others
References
Behr TM, Béhé M, Becker W. Diagnostic applications of radiolabeled peptides in nuclear endocrinology. Q J Nucl Med 1999; 43:268–280.
Breeman WAP, de Jong M, Kwekkeboom DJ, et al. Somatostatin receptor-mediated imaging and therapy: basic science, current knowledge, limitations and future perspectives. Eur J Nucl Med 2001; 28:1421–1429.
Fischman A, Babich J, Strauss H. A ticket to ride: peptide radiopharmaceuticals. J Nucl Med 1993; 34:2253–2263.
Heppeler A, Froidevaux S, Eberle A, Maecke H. Receptor targeting for tumor localization and therapy with radiopeptides. Curr Med Chem 2000; 7:971–994.
Lamberts SW, Van der Lely AJ, De Herder WW, Hofland LJ. Octreotide. N Engl J Med 1996; 25:246–254.
Lister-James J, Moyer B, Dean T. Small peptides radiolabeled with99mTc. Q J Nucl Med 1996; 40:221–233.
Liu S, Edwards D.99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev 1999; 99:2235–2268.
Reubi JC. Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 1995; 36:1825–1835.
Thakur M. Radiolabelled peptides: now and the future. Nucl Med Comm 1995; 16:724–732.
Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993; 20:716–731.
Gibril F, Reynolds JC, Doppman JL, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas—a prospective study. Ann Intern Med 1996; 125:24–26.
Blum JE, Handmaker H, Rinne NA. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. CHEST 1999; 115:224–232.
Virgolini I, Leimer M, Handmaker H, et al. Somatostatin receptor subtype specificity and in vivo binding of a novel tumor tracer,99mTc-P829. Cancer Res 1998; 58:1850–1859.
Lebtahi R, Le Cloirec J, Houzard C, et al. Detection of neuroendocrine tumors:99mTc-P829 scintigraphy compared with 111In-pentetreotide scintigraphy. J Nucl Med 2002; 43:889–895.
de Jong M, Bakker WH, Krenning EP, et al. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0,d-Phe1,Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997; 24:368–371.
Stolz B, Weckbecker G, Smith-Jones PM, Albert R, Raulf F, Bruns C. The somatostatin receptor-targeted radiotherapeutic [90Y-DPTA-dPhe1,Tyr3]octreotide (90Y-SMT 487) eradicates experimental rat pancreatic CA 20948 tumours. Eur J Nucl Med 1998; 25:668–674.
Otte A, Herrmann R, Heppeler A, et al. Yttrium-90-DOTATOC: first clinical results. Eur J Nucl Med 1999; 26:1439–1447.
Otte A, Mueller-Brand J, Dellas S, Nitzsche E, Herrmann R, Maecke H. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet 1998; 351:417–418.
Cremonesi M, Ferrari M, Zoboli S, et al. Biokinetics and dosimetry in patients administered with111In-DOTA-Tyr3-octreotide: implications for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med 1999; 26:877–886.
Waldherr C, Pless M, Maecke H, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq90Y-DOTATOC. J Nucl Med 2002; 43:610–616.
Paganelli G, Zoboli S, Cremonesi M, et al. Receptor-mediated radiotherapy with90Y-DOTA-Phe1-Tyr3-octreotide. Eur J Nucl Med 2001; 28:426–434.
Waldherr C, Pless M, Maecke H, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-d-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001; 12:942–945.
de Jong M, Breeman WA, Bernard BF, et al. Tumor response after [90Y-DOTA0,Tyr3]octreotide radionuclide therapy in a transplantable rat tumor model is dependent on tumor size. J Nucl Med 2001; 42:1841–1846.
Reubi J, Schaer J, Waser B, et al. Affinity profiles for human somatostatin receptor sst1–sst5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000; 27:273–282.
Kwekkeboom DJ, Bakker WH, Kooij PP, et al. [177Lu-DOTA0,Tyr3]octreotate: comparison with [111DTPA0]octreotide in patients. Eur J Nucl Med 2001; 28:1319–1325.
Reubi JC, Waser B, Schaer J, Laissue JA. Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001; 28:836–846.
Halmos G, Sun B, Schally AV, Hebert F, Nagy A. Human ovarian cancers express somatostatin receptors. J Clin Endocrinol Metab 2000; 85:3509–3512.
Reubi JC, Schaer J, Waser B, Mengod G. Expression and localization of somatostatin receptor sstr1, sstr2, and sstr3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res 1994; 54:3455–3459.
Forssell-Aronsson E, Nilsson O, Benjegard SA, et al.111In-DTPA-d-Phe1-octreotide binding and somatostatin receptor subtypes in thyroid tumors. J Nucl Med 2000; 41:636–642.
Raderer M, Pangerl T, Leimer M, et al. Expression of human somatostatin receptor subtype 3 in pancreatic cancer in vitro and in vivo. J Nat Cancer Inst 1998; 90:1666–1668.
Traub T, Petkov V, Ofluoglu S, et al.111In-DOTA-lanreotide scintigraphy in patients with tumors of the lung. J Nucl Med 2001; 42:1309–1315.
Heppeler A, Froidevaux S, Maecke HR, et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J 1999; 5:1974–1981.
Atherton E, Sheppard R. Fluorenylmethoxycarbonyl-polyamide solid phase peptide synthesis. General principles and development. Oxford: Oxford Information Press, 1989.
Oberg K. Established clinical use of octreotide and lanreotide in oncology. Chemotherapy 2001; 47:40–53.
Chiti A, Fanti S, Savelli G, et al. Comparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastro-entero-pancreatic tumours. Eur J Nucl Med 1998; 25:1396–1403.
Lebtahi R, Cadiot G, Sarda L, et al. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med 1997; 38:853–858.
Cadiot G, Bonnaud G, Lebtahi R, et al. Usefulness of somatostatin receptor scintigraphy in the management of patients with Zollinger-Ellison syndrome. Gut 1997; 41:107–114.
Termanini B, Gibril F, Reynolds JC, et al. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology 1997; 112:335–347.
Gibril F, Doppman JL, Reynolds JC, et al. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J Clin Oncol 1998; 16:1040–1053.
Krenning EP, de Jong M, Kooij PP, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol 1999; 10 (Suppl 2):S23–S29.
Janson E, Eriksson B, Oberg K. Treatment with high dose [111In-DTPA-d-Phe1]-octreotide in patients with neuroendocrine tumors. Acta Oncol 1999; 38:373–377.
Valkema R, de Jong M, Bakker WH, et al. Phase 1 study of peptide receptor radionuclide therapy with [111In-DTPA0]-octreotide: the Rotterdam experience. Semin Nucl Med 2002; 32:110–123.
de Jong M, Breeman WA, Bernard BF, et al. [177Lu-DOTA0,Tyr3]Octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer 2001; 92:628–633.
Lewis J, Lewis M, Srinivasan A, Schmidt MA, Wang J, Anderson CJ. Comparison of four64Cu-labeled somatostatin analogues in vitro and in tumor-bearing rat model: evaluation of new derivatives for positron emission tomography imaging and targeted radiotherapy. J Med Chem 1998; 42:1341–1347.
Froidevaux S, Heppeler A, Eberle A, et al. Preclinical comparison in AR4-2J tumor bearing-mice of four radiolabeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-somatostatin analogs for tumor diagnosis and internal radiotherapy. Endocrinology 2000; 141:3304–3312.
Reubi JC, Eisenwiener KP, Rink H, Waser B, Maecke H. A new peptide somatostatin agonist with high affinity to all five somatostatin receptors. Eur J Pharmacol 2002; 456:45–49.
Smith-Jones PM, Bischof C, Leimer M, et al. DOTA-lanreotide: a novel somatostatin analog for tumor diagnosis and therapy. Endocrinology 1999; 140:5136–5148.
Maecke H, Scherer G, Heppeler A, Hennig M. Is In-111 an ideal surrogate for Y-90? If not why? Eur J Nucl Med 2001; 28:967.
Eisenwiener KP, Prata MIM, Buschmann I, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconj Chem 2002; 13:530–541.
Koenig JA, Kaur R, Dodgeon I, Edwardson JM, Humphrey PPA. Fates of endocytosed somatostatin sst2 receptor and associated agonists. Biochem J 1998; 336:291–298.
Raulf F, Pérez J, Joyer D, Bruns C. Differential expression of five somatostatin receptor subtypes, sstr1-5, in the CNS and peripheral tissue. Digestion 1994; 55 (Suppl 3):46–53.
Acknowledgements
M. Ginj, D. Wild, J. Schmitt and H. Maecke acknowledge support from the Swiss National Science Foundation project No. 31-52969.97, BBW No. C00.0091 and BBT project 4668.1 EUS. The support provided by Novartis Pharma in respect of MS and NMR is gratefully acknowledged.
This work was performed within the COST B12 action.
Author information
Authors and Affiliations
Corresponding author
Additional information
Abbreviations of the common amino acids are in accordance with the recommendations of IUPAC-IUB [IUPAC-IUB Commission of Biochemical Nomenclature (CBN), Symbols for amino-acid derivatives and peptides, recommendations 1971. Eur J Biochem 1972; 27:201–207].
Rights and permissions
About this article
Cite this article
Wild, D., Schmitt, J.S., Ginj, M. et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging 30, 1338–1347 (2003). https://doi.org/10.1007/s00259-003-1255-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1255-5