Abstract
Objective
To determine if dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) parameters reflect histological grade of soft tissue sarcoma (STS)
Materials and methods
The medical records of 50 patients diagnosed with pathologically confirmed STS were retrospectively reviewed. Each STS was assessed with conventional contrast-enhanced MRI and DCE-MRI using a 3.0-T MRI system. The conventional MRI characteristics of low-grade (grade 1) and high-grade (grade 2 and grade 3) tumors were analyzed. Semi-quantitative parameters, including iAUC and TTP, and quantitative parameters, including Ktrans, Kep, and Ve, were derived from DCE-MRI. The diagnostic performances and optimal thresholds of various combinations of DCE-MRI parameters for predicting histological grades of STS were investigated using receiver operator characteristic (ROC) curves.
Results
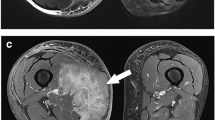
On conventional MRI, high-grade STSs were significantly larger (≥ 5 cm) and more likely to show a heterogeneous signal intensity on T2WI (> 75%), peritumoral hyperintensity on T2WI, or tumor necrosis (> 50%) compared with low-grade STS. On DCE-MRI, iAUC, TTP, Ktrans, and Kep were significant predictors of STS histological grade. Ktrans had a high diagnostic value for differentiating between high-grade and low-grade STSs. The combination of iAUC, TTP, and Ktrans yielded a higher AUC value (0.841) than the other models.
Conclusion
High-grade STSs were usually larger than low-grade STSs, had unclear boundaries, a heterogeneous signal intensity on T2-weighted image (T2WI), and extensive necrosis. On DCE-MRI, iAUC, TTP, Ktrans, and Kep could differentiate between high-grade and low-grade STSs. The combination of iAUC, TTP, and Ktrans had a high diagnostic performance for differentiating between STS histological grades.






Similar content being viewed by others
References
Gronchi A, Ferrari S, Quagliuolo V, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–22.
Issels RD, Lindner LH, Verweij J, et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol. 2010;11(6):561–70.
Lucas DR, Kshirsagar MP, Biermann JS, et al. Histologic alterations from neoadjuvant chemotherapy in high-grade extremity soft tissue sarcoma: clinicopathological correlation. Oncologist. 2008;13(4):451–8.
Menendez LR, Ahlmann ER, Savage K, Cluck M, Fedenko AN. Tumor necrosis has no prognostic value in neoadjuvant chemotherapy for soft tissue sarcoma. Clin Orthop Relat Res. 2007;455:219–24.
Pasquali S, Gronchi A. Neoadjuvant chemotherapy in soft tissue sarcomas: latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(6):415–29.
Saponara M, Stacchiotti S, Casali PG, Gronchi A. (Neo)adjuvant treatment in localised soft tissue sarcoma: the unsolved affair. Eur J Cancer. 2017;70:1–11.
Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869–77.
Guillou L, Coindre JM, Bonichon F, et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol. 1997;15(1):350–62.
Coindre JM, Terrier P, Guillou L, et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–26.
Zagars GK, Ballo MT, Pisters PW, et al. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: an analysis of 1225 patients. Cancer. 2003;97(10):2530–43.
Atalar H, Basarir K, Yildiz Y, Saglik Y. Prognostic factors in patients with malignant fibrous histiocytoma of the extremities. Acta Orthop Traumatol Turc. 2007;41(4):271–6.
Crombe A, Marcellin PJ, Buy X, et al. Soft-tissue sarcomas: assessment of MRI features correlating with histologic grade and patient outcome. Radiology. 2019;291(3):710–21.
Yang J, Frassica FJ, Fayad L, Clark DP, Weber KL. Analysis of nondiagnostic results after image-guided needle biopsies of musculoskeletal lesions. Clin Orthop Relat Res. 2010;468(11):3103–11.
Noebauer-Huhmann IM, Weber MA, Lalam RK, et al. Soft tissue tumors in adults: ESSR-approved guidelines for diagnostic imaging. Semin Musculoskelet Radiol. 2015;19(5):e1.
Barrientos-Ruiz I, Ortiz-Cruz EJ, Serrano-Montilla J, Bernabeu-Taboada D, Pozo-Kreilinger JJ. Are biopsy tracts a concern for seeding and local recurrence in sarcomas? Clin Orthop Relat Res. 2017;475(2):511–8.
Siddiqi MA, Kim HS, Jede F, Han I. Association of core needle biopsy tract resection with local recurrence in extremity soft tissue sarcoma. Skelet Radiol. 2017;46(4):507–12.
Van Houdt WJ, Schrijver AM, Cohen-Hallaleh RB, et al. Needle tract seeding following core biopsies in retroperitoneal sarcoma. Eur J Surg Oncol. 2017;43(9):1740–5.
Chhabra A, Ashikyan O, Slepicka C, et al. Conventional MR and diffusion-weighted imaging of musculoskeletal soft tissue malignancy: correlation with histologic grading. Eur Radiol. 2019;29(8):4485–94.
Fayad LM, Mugera C, Soldatos T, Flammang A, del Grande F. Technical innovation in dynamic contrast-enhanced magnetic resonance imaging of musculoskeletal tumors: an MR angiographic sequence using a sparse k-space sampling strategy. Skelet Radiol. 2013;42(7):993–1000.
Noebauer-Huhmann IM, Amann G, Krssak M, et al. Use of diagnostic dynamic contrast-enhanced (DCE)-MRI for targeting of soft tissue tumour biopsies at 3T: preliminary results. Eur Radiol. 2015;25(7):2041–8.
Xia W, Yan Z, Gao X. Volume fractions of DCE-MRI parameter as early predictor of histologic response in soft tissue sarcoma: a feasibility study. Eur J Radiol. 2017;95:228–35.
Choi YJ, Lee IS, Song YS, Kim JI, Choi KU, Song JW. Diagnostic performance of diffusion-weighted (DWI) and dynamic contrast-enhanced (DCE) MRI for the differentiation of benign from malignant soft-tissue tumors. J Magn Reson Imaging. 2019;50(3):798–809.
Egeland TA, Gulliksrud K, Gaustad JV, Mathiesen B, Rofstad EK. Dynamic contrast-enhanced-MRI of tumor hypoxia. Magn Reson Med. 2012;67(2):519–30.
O'Connor JP, Jackson A, Parker GJ, Roberts C, Jayson GC. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol. 2012;9(3):167–77.
Alic L, van Vliet M, Wielopolski PA, et al. Regional heterogeneity changes in DCE-MRI as response to isolated limb perfusion in experimental soft-tissue sarcomas. Contrast Media Mol Imaging. 2013;8(4):340–9.
Lee JH, Yoon YC, Seo SW, Choi YL, Kim HS. Soft tissue sarcoma: DWI and DCE-MRI parameters correlate with Ki-67 labeling index. Eur Radiol. 2020;30(2):914–24.
Reynoso D, Subbiah V, Trent JC, et al. Neoadjuvant treatment of soft-tissue sarcoma: a multimodality approach. J Surg Oncol. 2010;101(4):327–33.
Sundby Hall K, Bruland OS, Bjerkehagen B, et al. Adjuvant chemotherapy and postoperative radiotherapy in high-risk soft tissue sarcoma patients defined by biological risk factors-a Scandinavian Sarcoma Group study (SSG XX). Eur J Cancer. 2018;99:78–85.
Zhao F, Ahlawat S, Farahani SJ, et al. Can MR imaging be used to predict tumor grade in soft-tissue sarcoma? Radiology. 2014;272(1):192–201.
Lefkowitz RA, Landa J, Hwang S, et al. Myxofibrosarcoma: prevalence and diagnostic value of the “tail sign” on magnetic resonance imaging. Skelet Radiol. 2013;42(6):809–18.
Lee AY, Agaram NP, Qin LX, et al. Optimal percent myxoid component to predict outcome in high-grade myxofibrosarcoma and undifferentiated pleomorphic sarcoma. Ann Surg Oncol. 2016;23(3):818–25.
Kim SH, Choi MS, Kim MJ, Kim YH, Cho SH. Role of semi-quantitative dynamic contrast-enhanced MR imaging in characterization and grading of prostate cancer. Eur J Radiol. 2017;94:154–9.
Chen J, Chen C, Xia C, et al. Quantitative free-breathing dynamic contrast-enhanced MRI in hepatocellular carcinoma using gadoxetic acid: correlations with Ki67 proliferation status, histological grades, and microvascular density. Abdom Radiol (NY). 2018;43(6):1393–403.
Koo HR, Cho N, Song IC, et al. Correlation of perfusion parameters on dynamic contrast-enhanced MRI with prognostic factors and subtypes of breast cancers. J Magn Reson Imaging. 2012;36(1):145–51.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Mills SJ, Soh C, Rose CJ, et al. Candidate biomarkers of extravascular extracellular space: a direct comparison of apparent diffusion coefficient and dynamic contrast-enhanced MR imaging--derived measurement of the volume of the extravascular extracellular space in glioblastoma multiforme. AJNR Am J Neuroradiol. 2010;31(3):549–53.
Li SP, Padhani AR, Taylor NJ, et al. Vascular characterisation of triple negative breast carcinomas using dynamic MRI. Eur Radiol. 2011;21(7):1364–73.
Jansen JF, Carlson DL, Lu Y, et al. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: initial analysis. Oral Oncol. 2012;48(8):717–22.
Li X, Zhu Y, Kang H, et al. Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging. 2015;15:4.
Jia Z, Geng D, Xie T, Zhang J, Liu Y. Quantitative analysis of neovascular permeability in glioma by dynamic contrast-enhanced MR imaging. J Clin Neurosci. 2012;19(6):820–3.
Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7(1):91–101.
Khalifa F, Soliman A, El-Baz A, et al. Models and methods for analyzing DCE-MRI: a review. Med Phys. 2014;41(12):124301.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of our institution, which waived the need to obtain written informed consent from the included patients.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., Wang, Q., Dou, Y. et al. Soft tissue sarcoma: can dynamic contrast-enhanced (DCE) MRI be used to predict the histological grade?. Skeletal Radiol 49, 1829–1838 (2020). https://doi.org/10.1007/s00256-020-03491-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03491-z