Abstract
Background
Preoperative diffusion-weighted MRI (DW-MRI) has been described as an efficient method to differentiate good and poor responders to chemotherapy in osteosarcoma patients. A DW-MRI performed earlier during treatment could be helpful in monitoring chemotherapy.
Objective
To assess the accuracy of DW-MRI in evaluating response to chemotherapy in the treatment of osteosarcoma, more specifically at mid-course of treatment.
Materials and methods
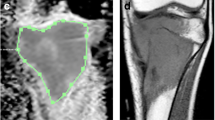
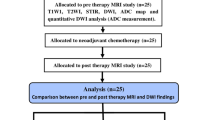
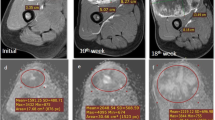
This study was carried out on a prospective series of adolescents treated for long-bone osteosarcoma. MR examinations were performed at diagnosis (MRI-1), at mid-course of chemotherapy (MRI-2), and immediately before surgery (MRI-3). A DW sequence was performed using diffusion gradients of b0 and b900. The apparent diffusion coefficients (ADC1, ADC2, ADC3, respectively), their differentials (ADC2 − ADC1 and ADC3 − ADC1), and their variation (ADC2 − ADC1/ADC1 and ADC3 − ADC1/ADC1) were calculated for each of these three time points.
Results
Fifteen patients were included. Patients with no increase in ADC showed a poor response to chemotherapy on their histology results. At mid-course, the three calculated values were significantly different between good and poor responders. ADC2 − ADC1 enabled us to detect, with 100% specificity, four out of seven of the poor responders. There was no significant difference in the values at MRI-3 between the two groups.
Conclusion
DW-MRI performed both at baseline and mid-course of neoadjuvant chemotherapy is an efficient method to predict further histological response of osteosarcoma. This method could be used as an early prognostic factor to monitor preoperative chemotherapy.



Similar content being viewed by others
References
Bacci G, Longhi A, Versari M, et al. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvantchemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer 2006;106(5):1154–6
Le Deley MC, Guinebretière JM, Gentet JC, Société Française d’Oncologie Pédiatrique (SFOP), et al. SFOP OS94: a randomised trial comparing preoperative high-dose methotrexate plus doxorubicin to high-dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. Eur J Cancer. 2007;43(4):752–61.
Kim MS, Lee SY, Cho WH, et al. Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol. 2008;97(5):456-61
Bajpai J, Gamnagatti S, Kumar R, et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol. 2010;41(4):441–50.
Bramer JA, van Linge JH, Grimer RJ, et al. Prognostic factors in localized extremity osteosarcoma: a systematic review. Eur J Surg Oncol. 2009;35(10):1030–6.
Brisse H, Ollivier L, Edeline V, et al. Imaging of malignant tumours of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol. 2004;34:595–605.
Pan G, Raymond AK, Carrasco CH, Wallace S, et al. Osteosarcoma: MR imaging after preoperative chemotherapy. Radiology. 1990;174:517–26.
Holscher HC, Bloem JL, Vanel D, et al. Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology. 1992;182:839–44.
Van der Woude HJ, Bloem JL, Verstraete KL, et al. Osteosarcoma and Ewing’s sarcoma after neoadjuvent chemotherapy: value of dynamic MR Imaging in detecting viable tumor before surgery. AJR Am J Roentgenol. 1995;165:593–8.
Verstraete KL, Van der Woude HJ, et al. Dynamic contrast-enhanced MR imaging of musculoskeletal tumors: basic principles and clinical applications. J Magn Reson Imaging. 1996;6:311–21.
Reddick WE, Wang S, Xiong X, et al. Dynamic magnetic resonance Imaging of regional contrast access as an additional prognostic factor in pediatric osteosarcoma. Cancer. 2001;91(12):2230–7.
Dyke JP, Panicek DM, Healey JH, et al. Osteogenic and Ewing sarcomas: estimation of necrotic fraction during induction chemotherapy with dynamic contrast- enhanced MR Imaging. Radiology. 2003;228:271–8.
Uhl M, Saueressig U, van Buiren M, Kontny U, et al. Osteosarcoma: preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol. 2006;41(8):618–23.
Ongolo-Zogo P, Thiesse P, Sau J, Desuvinges C, et al. Assessment of osteosarcoma response to neoadjuvent chemotherapy: comparative usefulness of dynamic gadolinium-enhanced spin-echo magnetic resonance imaging and technetium-99 m skeletal angioscintigraphy. Eur Radiol. 1999;9:907–14.
Van Rijswijk CSP, Kunz P, Hogendoorn PCW, et al. Diffusion-weighted MRI in the characterization of soft-tissue tumors. J Magn Reson Imaging. 2002;15:302–7.
Baur A, Huber A, Arbogast S, et al. Diffusion-weighted imaging of tumor recurrencies and posttherapeutical soft-tissue changes in humans. Eur Radiol. 2001;11:828–33.
Baur A, Reiser MF. Diffusion-weighted imaging of the musculoskeletal system in humans. Skeletal Radiol. 2000;29:555–62.
Herneth AM, Friedrich K, Weidekamm C, Schibany N, et al. Diffusion weighted imaging of bone marrow pathologies. Eur J Radiol. 2005;55:74–83.
MacKenzie JD, Gonzalez L, Hernandez A. Diffusion-weighted and diffusion tensor imaging for pediatric musculoskeletal disorders. Pediatr Radiol. 2007;37:781–8.
Nonomura Y, Yasumoto M, Yoshimura R, Haraguchi K, et al. Relationship between bone marrow cellularity and apparent diffusion coefficient. J Magn Reson Imaging. 2001;13:757–60.
Humphries PD, Sebire NJ, Siegel MJ, et al. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology. 2007;245(3):848–54.
Lang P, Wendland MF, Saeed M, Gindele A, Rosenau W. Osteogenic osteosarcoma: non-invasive in vivo assessment of tumor necrosis with diffusion-weighted MR imaging. Radiology. 1998;206:227–35.
Thoeny HC, De Keyser F, Chen F. Diffusion-weighted MR imaging in monitoring the effect of a vascular targeting agent on rhabdomyosarcoma in rats. Radiology. 2005;234:756–64.
Uhl M, Saueressig U, Koehler G. Evaluation of tumour necrosis during chemotherapy with diffusion-weighted MR imaging: preliminary results in osteosarcomas. Pediatr Radiol. 2006;36:1306–11.
Hayashida Y, Yakushiji T, Awai K. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted images: initial results. Eur Radiol. 2006;16:2637–43.
Oka K, Yakushiji T, Sato H. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol. 2010;39(2):141–6.
Denecke T, Hundsdörfer P, Misch D, et al. Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging. 2010;37(10):1842–53.
Costelloe CM, Raymond AK, Fitzgerald NE, et al. Tumor necrosis in osteosarcoma: inclusion of the point of greatest metabolic activity from F-18 FDG PET/CT in the histopathologic analysis. Skeletal Radiol. 2010;39(2):131–40.
Cheon GJ, Kim MS, Lee JA, et al. Prediction model of chemotherapy response in osteosarcoma by 18 F-FDG PET and MRI. J Nucl Med. 2009;50(9):1435–40.
Hawkins DS, Conrad EU 3rd, Butrynski JE. [F-18]-fluorodeoxy-D-glucose-positron emission tomography response is associated with outcome for extremity osteosarcoma in children and young adults. Cancer. 2009;115(15):3519-25.
Costelloe CM, Macapinlac HA, Madewell JE, et al. 18 F-FDG PET/CT as an indicator of progression-free and overall survival in osteosarcoma. J Nucl Med. 2009;50(3):340–7.
Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101(1):14–8.
Parker GJ. Analysis of MR diffusion weighted images. Br J Radiol. 2004;77:S176–85.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baunin, C., Schmidt, G., Baumstarck, K. et al. Value of diffusion-weighted images in differentiating mid-course responders to chemotherapy for osteosarcoma compared to the histological response: preliminary results. Skeletal Radiol 41, 1141–1149 (2012). https://doi.org/10.1007/s00256-012-1360-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-012-1360-2