Abstract
Objective
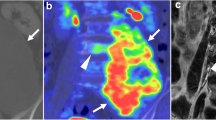
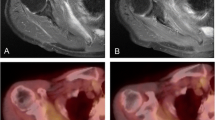
Chordoma is a rare malignant bone tumor that arises from notochord remnants. This is the first trial to investigate the utility of 11C-methionine (MET) positron emission tomography (PET) in the imaging of chordoma before and after carbon-ion radiotherapy (CIRT).
Design and patients
Fifteen patients with chordoma were investigated with MET-PET before and after CIRT and the findings analyzed visually and quantitatively. Tumor MET uptake was evaluated by tumor-to-nontumor ratio (T/N ratio).
Results
In 12 (80%) patients chordoma was clearly visible in the baseline MET-PET study with a mean T/N ratio of 3.3±1.7. The MET uptake decreased significantly to 2.3±1.4 after CIRT (P<0.05). A significant reduction in tumor MET uptake of 24% was observed after CIRT. Fourteen (93%) patients showed no local recurrence after CIRT with a median follow-up time of 20 months.
Conclusion
This study has demonstrated that MET-PET is feasible for imaging of chordoma. MET-PET could provide important tumor metabolic information for the therapeutic monitoring of chordoma after CIRT.




Similar content being viewed by others
References
Baratti D, Gronchi A, Pennacchioli E, et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol 2003; 10:291-296.
Mindell ER. Chordoma. J Bone Joint Surg Am 1981; 63:501–505.
Breteau N, Demasure M, Lescrainier J, Sabbattier R, Michenet P. Sacrococcygeal chordomas: potential role of high LET therapy. Recent Results Cancer Res 1998; 150:148-155.
Watkins L, Khudados ES, Kaleoglu M, Revesz T, Sacares P, Crockard HA. Skull base chordomas: a review of 38 patients, 1958–88. Br J Neurosurg 1993; 7:241-248.
Gay E, Sekhar LN, Rubinstein E, et al. Chordomas and chondrosarcomas of the cranial base: results and follow-up of 60 patients. Neurosurgery 1995; 36:887-897.
Al-Mefty O, Borba LA. Skull base chordomas: a management challenge. J Neurosurg 1997; 86:182-189.
Krayenbuhl H, Yasargil MG. Cranial chordomas. Progr Neurol Surg 1975; 6:380–434.
Benk V, Liebsch NJ, Munzenrider JE, Efird J, McManus P, Suit H. Base of skull and cervical spine chordomas in children treated by high-dose irradiation. Int J Radiat Oncol Biol Phys 1995; 31:577–581.
Castro JR, Collier JM, Petti PL, et al. Charged particle radiotherapy for lesions encircling the brain stem or spinal cord. Int J Radiat Oncol Biol Phys 1989; 17:477–484.
Kamada T, Tsujii H, Tsuji H, et al. Working Group for the Bone and Soft Tissue Sarcomas. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol 2002; 20:4466–4471.
Gambhir SS. Molecular imaging of cancer with positron emission tomography. Natl Rev Cancer 2002; 2:683–693.
Zhang H, Tian M, Oriuchi N, Higuch, T, Tanada S, Endo K. Oncological diagnosis using positron coincidence gamma camera with fluorodeoxyglucose in comparison with dedicated PET. Br J Radiol 2002; 75:409–416.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992; 33:1972―1980.
Haberkorn U, Strauss LG, Dimitrakopoulou A, et al. PET studies of fluorodeoxyglucose metabolism in patients with recurrent colorectal tumors receiving radiotherapy. J Nucl Med 1991; 32:1485–1490.
Hautzel H, Muller-Gartne, HW. Early changes in fluorine-18-FDG uptake during radiotherapy. J Nucl Med 1997; 38:1384–1386.
Hoffman RM. Unbalanced transmethylation and the perturbation of the differentiated state leading to cancer. Bioessays 1990; 12:163–166.
Kracht LW, Friese M, Herholz K, et al. Methyl-[11C]-l-methionine uptake as measured by positron emission tomography correlates to microvessel density in patients with glioma. Eur J Nucl Med Mol Imaging 2003; 30:868―873.
Kubota R, Kubota K, Yamada S, et al. Methionine uptake by tumor tissue: a microautoradiographic comparison with FDG. J Nucl Med 1995; 36:484–492.
Schaider H, Haberkorn U, Berger MR, Oberdorfer F, Morr I, van Kaick G. Application of alpha-aminoiosbutyric acid,l-methionine, thymidine and 2-fluoro-2-d-glucose to monitor effects of chemotherapy in a human colon carcinoma cell line. Eur J Nucl Med 1996; 23:55–60.
Higashi K, Clavo AC, Wahl RL. In vitro assessment of 2-fluoro-2-d-glucose, l-methionine, thymidine as agents to monitor the early response of a human adenocarcinoma cell line to radiotherapy. J Nucl Med 1993; 34:773–779.
Kubota K, Ishiwata K, Kubota R, et al. Tracer feasibility for monitoring tumor radiotherapy: a quadruple tracer study with fluorine-18-fluorodeoxyglucose or fluorine-18-fluorodeoxyuridine,l-[methyl-14C] methionine, [6-3H]thymidine, and gallium-67. J Nucl Med 1991; 32:2118–2123.
Minn H, Clavo AC, Grenman R, Wahl RL. In vitro comparison of cell proliferation kinetics and uptake of tritiated fluorodeoxyglucose andl-methionine in squamous-cell carcinoma of the head and neck. J Nucl Med 1‘995; 36:252–258.
Stern PH, Hoffman RM. Elevated overall rates of transmethylation in cell lines from diverse human tumors. In Vitro 1984; 20:663–670.
Stern PH, Wallace CD, Hoffman RM. Altered methionine metabolism occurs in all members of a set of diverse human tumor cell lines. J Cell Physiol 1984; 119:29–34.
Wheatley DN. On the problem of linear incorporation of amino acids into cell protein. Experientia 1982; 38:818–820.
Weiss SW. WHO international histological classification of tumours. Histological typing of soft tissue tumours, 2nd edn. Berlin Heidelberg New York: Springer, 1994.
Langstrom B, Antoni G, Gullberg P, et al. Synthesis ofl-and d-[methy-11C]methionine. J Nucl Med 1987; 19:1037–1040.
Leskinen-Kallio S, Nagren K, Lehikoinen P, Ruotsalainen U, Joensuu H. Uptake of11C-methionine in breast cancer studied by PET. An association with the size of S-phase fraction. Br J Cancer 1991; 64:1121―1124.
Munzenrider JE, Liebsch NJ. Proton therapy for tumors of the skull base. Strahlenther Onkol 1999;175(Suppl 2):57–63.
Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg 1999; 91:432–439.
Verstraete KL, Lang P. Post-therapeutic magnetic resonance imaging of bone tumors. Top Magn Reson Imaging 1999; 10:237–246.
Fletcher BD. Effects of pediatric cancer therapy on the musculoskeletal system. Pediatr Radiol 1997; 27:623–636.
Murphy WA Jr. Imaging bone tumors in the 1990s. Cancer 1991; 67:1169–1176.
Hawkins DS, Rajendran JG, Conrad EU 3rd, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-d-glucose positron emission tomography. Cancer 2002; 94:3277–3284.
Findlay M, Young H, Cunningham D, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol 1996; 14:700–708.
Muhr C, Gudjonsson O, Lilja A, Hartman M, Zhang ZJ, Langstrom B. Meningioma treated with interferon-alpha, evaluated with [11C]-l-methionine positron emission tomography. Clin Cancer Res 2001; 7:2269―2276.
Andersson T, Wilander E, Eriksson B, Lindgren PG, Oberg K. Effects of interferon on tumor tissue content in liver metastases of human carcinoid tumors. Cancer Res 1990; 50:3413―3415.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was part of the 10-Year Strategy for Cancer Control of the Japanese Government and was supported by the Ministry of Education, Science and Technology of Japan Government
Rights and permissions
About this article
Cite this article
Zhang, H., Yoshikawa, K., Tamura, K. et al. Carbon-11-methionine positron emission tomography imaging of chordoma. Skeletal Radiol 33, 524–530 (2004). https://doi.org/10.1007/s00256-004-0815-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-004-0815-5