Abstract
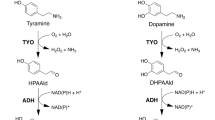
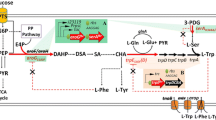
We have constructed an Escherichia coli-based platform producing (S)-reticuline, an important intermediate of benzylisoquinoline alkaloids (BIAs), using up to 14 genes. (S)-reticuline was produced from a simple carbon source such as glucose and glycerol via l-DOPA, which is synthesized by hydroxylation of l-tyrosine, one of the rate-limiting steps of the reaction. There are three kinds of enzymes catalyzing tyrosine hydroxylation: tyrosinase (TYR), tyrosine hydroxylase (TH), and 4-hydroxyphenylacetate 3-monooxygenase (HpaBC). Here, to further improve (S)-reticuline production, we chose eight from these three kinds of tyrosine hydroxylation enzymes (two TYRs, four THs, and two HpaBCs) derived from various organisms, and examined which enzyme was optimal for (S)-reticuline production in E. coli. TH from Drosophila melanogaster was the most suitable for (S)-reticuline production under the experimental conditions tested. We improved the productivity by genome integration of a gene set for l-tyrosine overproduction, introducing the regeneration pathway of BH4, a cofactor of TH, and methionine addition to enhance the S-adenosylmethionine supply. As a result, the yield of (S)-reticuline reached up to 384 μM from glucose in laboratory-scale shake flask. Furthermore, we found three inconsistent phenomena: an inhibitory effect due to additional gene expression, conflicts among the experimental conditions, and interference of an upstream enzyme from an additional downstream enzyme. Based on these results, we discuss future perspectives and challenges of integrating multiple enzyme genes for material production using microbes.

The optimal tyrosine hydroxylation enzyme for (S)-reticuline production in Escherichia coli
Key points
• There are three types of enzymes catalyzing tyrosine hydroxylation reaction: tyrosinase, tyrosine hydroxylase, and 4-hydroxyphenylacetate 3-monooxygenase.
• Tyrosine hydroxylase from Drosophila melanogaster exhibited the highest activity and was suitable for (S)-reticuline production in E. coli.
• New insights were provided on constructing an alkaloid production system with multi-step reactions in E. coli.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Bhattacharyya S, Bershtein S, Yan J, Argun T, Gilson AI, Trauger SA, Shakhnovich EI (2016) Transient protein-protein interactions perturb E. coli metabolome and cause gene dosage toxicity. elife 5:e20309
Causey TB, Zhou S, Shanmugam KT, Ingram LO (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc Natl Acad Sci U S A 100(3):825–832
Das A, Tyagi N, Verma A, Akhtar S, Mukherjee KJ (2018) Metabolic engineering of Escherichia coli W3110 strain by incorporating genome-level modifications and synthetic plasmid modules to enhance L-Dopa production from glycerol. Prep Biochem Biotechnol 48(8):671–682
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645
DeLoache WC, Russ ZN, Narcross L, Gonzales AM, Martin VJ, Dueber JE (2015) An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat Chem Biol 11(7):465–471
Fordjour E, Adipah FK, Zhou S, Du G, Zhou J (2019) Metabolic engineering of Escherichia coli BL21 (DE3) for de novo production of L-DOPA from D-glucose. Microb Cell Factories 18(1):74
Furuya T, Kino K (2014) Catalytic activity of the two-component flavin-dependent monooxygenase from Pseudomonas aeruginosa toward cinnamic acid derivatives. Appl Microbiol Biotechnol 98(3):1145–1154
Hawkins KM, Smolke CD (2008) Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae. Nat Chem Biol 4(9):564–573
Hernández-Romero D, Sanchez-Amat A, Solano F (2006) A tyrosinase with an abnormally high tyrosine hydroxylase/dopa oxidase ratio. FEBS J 273(2):257–270
Karković Marković A, Torić J, Barbarić M, Jakobušić Brala C (2019) Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 24(10):2001
Kohashi PY, Kumagai T, Matoba Y, Yamamoto A, Maruyama M, Sugiyama M (2004) An efficient method for the overexpression and purification of active tyrosinase from Streptomyces castaneoglobisporus. Protein Expr Purif 34(2):202–207
Koma D, Kishida T, Yamanaka H, Moriyoshi K, Nagamori E, Ohmoto T (2018) Escherichia coli chromosome-based T7-dependent constitutive overexpression system and its application to generating a phenylalanine producing strain. J Biosci Bioeng 126(5):586–595
Lütke-Eversloh T, Stephanopoulos G (2007) L-tyrosine production by deregulated strains of Escherichia coli. Appl Microbiol Biotechnol 75(1):103–110
Lütke-Eversloh T, Santos CN, Stephanopoulos G (2007) Perspectives of biotechnological production of L-tyrosine and its applications. Appl Microbiol Biotechnol 77(4):751–762
Matsumura E, Nakagawa A, Tomabechi Y, Ikushiro S, Sakaki T, Katayama T, Yamamoto K, Kumagai H, Sato F, Minami H (2018) Microbial production of novel sulphated alkaloids for drug discovery. Sci Rep 8(1):7980
Minami H, Kim JS, Ikezawa N, Takemura T, Katayama T, Kumagai H, Sato F (2008) Microbial production of plant benzylisoquinoline alkaloids. Proc Natl Acad Sci U S A 105(21):7393–7398
Miró-Casas E, Covas MI, Fitó M, Farré-Albadalejo M, Marrugat J, de la Torre R (2003) Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur J Clin Nutr 57(1):186–190
Munoz AJ, Hernandez-Chavez G, de Anda R, Martinez A, Bolivar F, Gosset G (2011) Metabolic engineering of Escherichia coli for improving L-3,4-dihydroxyphenylalanine (L-DOPA) synthesis from glucose. J Ind Microbiol Biotechnol 38(11):1845–1852
Nakagawa A, Minami H, Kim JS, Koyanagi T, Katayama T, Sato F, Kumagai H (2011) A bacterial platform for fermentative production of plant alkaloids. Nat Commun 2:326
Nakagawa A, Matsuzaki C, Matsumura E, Koyanagi T, Katayama T, Yamamoto K, Sato F, Kumagai H, Minami H (2014) (R,S)-tetrahydropapaveroline production by stepwise fermentation using engineered Escherichia coli. Sci Rep 4:6695
Nakagawa A, Matsumura E, Koyanagi T, Katayama T, Kawano N, Yoshimatsu K, Yamamoto K, Kumagai H, Sato F, Minami H (2016) Total biosynthesis of opiates by stepwise fermentation using engineered Escherichia coli. Nat Commun 7:10390
Prieto MA, Garcia JL (1994) Molecular characterization of 4-hydroxyphenylacetate 3-hydroxylase of Escherichia coli. A two-protein component enzyme. J Biol Chem 269(36):22823–22829
Pyne ME, Kevvai K, Grewal PS, Narcross L, Choi B, Bourgeois L, Dueber JE, Martin VJJ (2019) A yeast platform for high-level synthesis of natural and unnatural tetrahydroisoquinoline alkaloids. bioRxiv:863506; https://doi.org/10.1101/863506
Pyne ME, Kevvai K, Grewal PS, Narcross L, Choi B, Bourgeois L, Dueber JE, Martin VJJ (2020) A yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids. Nat Commun 11(1):3337
Ribeiro P, Wang Y, Citron BA, Kaufman S (1992) Regulation of recombinant rat tyrosine hydroxylase by dopamine. Proc Natl Acad Sci U S A 89(20):9593–9597
Satoh Y, Tajima K, Munekata M, Keasling JD, Lee TS (2012) Engineering of L-tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol. Metab Eng 14(6):603–610
Sunnadeniya R, Bean A, Brown M, Akhavan N, Hatlestad G, Gonzalez A, Symonds VV, Lloyd A (2016) Tyrosine hydroxylation in betalain pigment biosynthesis is performed by cytochrome P450 enzymes in beets (Beta vulgaris). PLoS One 11(2):e0149417
Trenchard IJ, Siddiqui MS, Thodey K, Smolke CD (2015) De novo production of the key branch point benzylisoquinoline alkaloid reticuline in yeast. Metab Eng 31:74–83
Vié A, Cigna M, Toci R, Birman S (1999) Differential regulation of Drosophila tyrosine hydroxylase isoforms by dopamine binding and cAMP-dependent phosphorylation. J Biol Chem 274(24):16788–16795
Wei T, Cheng BY, Liu JZ (2016) Genome engineering Escherichia coli for L-DOPA overproduction from glucose. Sci Rep 6:30080
Yamamoto K, Kataoka E, Miyamoto N, Furukawa K, Ohsuye K, Yabuta M (2003) Genetic engineering of Escherichia coli for production of tetrahydrobiopterin. Metab Eng 5(4):246–254
Acknowledgements
Professor Taek Soon Lee (Lawrence Berkeley National Laboratory) kindly gifted PCD and DHPR genes. We thank Dr. Yasuharu Satoh (Hokkaido University) for advice and discussion.
Funding
This work was supported by the Project P16009, Development of Production Techniques for Highly Functional Biomaterials Using Smart Cells of Plants and Other Organisms (Smart Cell Project), from the New Energy and Industrial Technology Development Organization (NEDO to H.M.). This work was partly supported by the “Science and Technology that Create New Industries” of the Cannon Foundation and Grant-Support of Asahi Glass Foundation (to A.N.).
Author information
Authors and Affiliations
Contributions
A.N. and H.M. conceived and designed all experiments. A.N., S.N., E.M., Y.Y., and M.T. performed the experiments. S.A. and K.Y. chose the candidate enzymes. A.N, H.M, and T.K. discussed the results. A.N., T.K., and H.M. wrote the manuscript. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human or animal subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 787 kb)
Rights and permissions
About this article
Cite this article
Nakagawa, A., Nakamura, S., Matsumura, E. et al. Selection of the optimal tyrosine hydroxylation enzyme for (S)-reticuline production in Escherichia coli. Appl Microbiol Biotechnol 105, 5433–5447 (2021). https://doi.org/10.1007/s00253-021-11401-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11401-z