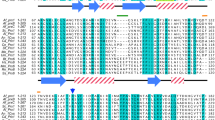
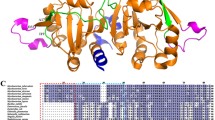
Abstract
The oxidative and nitrosative responses generated by animals and plants are important defenses against infection and establishment of pathogenic microorganisms such as bacteria, fungi, and protozoa. Among distinct oxidant species, hydroperoxides are a group of chemically diverse compounds that comprise small hydrophilic molecules, such as hydrogen peroxide and peroxynitrite, and bulky hydrophobic species, such as organic hydroperoxides. Peroxiredoxins (Prx) are ubiquitous enzymes that use a highly reactive cysteine residue to decompose hydroperoxides and can also perform other functions, like molecular chaperone and phospholipase activities, contributing to microbial protection against the host defenses. Prx are present in distinct cell compartments and, in some cases, they can be secreted to the extracellular environment. Despite their high abundance, Prx expression can be further increased in response to oxidative stress promoted by host defense systems, by treatment with hydroperoxides or by antibiotics. In consequence, some isoforms have been described as virulence factors, highlighting their importance in pathogenesis. Prx are very diverse and are classified into six different classes (Prx1-AhpC, BCP-PrxQ, Tpx, Prx5, Prx6, and AhpE) based on structural and biochemical features. Some groups are absent in hosts, while others present structural peculiarities that differentiate them from the host’s isoforms. In this context, the intrinsic characteristics of these enzymes may aid the development of new drugs to combat pathogenic microorganisms. Additionally, since some isoforms are also found in the extracellular environment, Prx emerge as attractive targets for the production of diagnostic tests and vaccines.
Key points
• Peroxiredoxins are front-line defenses against host oxidative and nitrosative stress.
• Functional and structural peculiarities differ pathogen and host enzymes.
• Peroxiredoxins are potential targets to microbicidal drugs.





Similar content being viewed by others
References
Ahn J, Jang KK, Jo I, Nurhasni H, Lim JG, Yoo J-W, Choi SH, Ha N-C, Ahn J, Jang KK, Jo I, Nurhasni H, Lim JG, Yoo J-W, Choi SH, Ha N-C (2018) Crystal structure of peroxiredoxin 3 from and its implications for scavenging peroxides and nitric oxide. IUCrJ 5(1):82–92
Akerman SE, Muller S (2005) Peroxiredoxin-linked detoxification of hydroperoxides in Toxoplasma gondii. J Biol Chem 280(1):564–570. https://doi.org/10.1074/jbc.M406367200
Aljannat MAK, Oldfield NJ, Albasri HM, Dorrington LKG, Ohri RL, Wooldridge KG, Turner DPJ (2020) The moonlighting peroxiredoxin-glutaredoxin in Neisseria meningitidis binds plasminogen via a C-terminal lysine residue and contributes to survival in a whole blood model. Microb Pathog 139:103890. https://doi.org/10.1016/j.micpath.2019.103890
Andrade HM, Murta SM, Chapeaurouge A, Perales J, Nirde P, Romanha AJ (2008) Proteomic analysis of Trypanosoma cruzi resistance to Benznidazole. J Proteome Res 7(6):2357–2367. https://doi.org/10.1021/pr700659m
Angelucci F, Miele AE, Ardini M, Boumis G, Saccoccia F, Bellelli A (2016) Typical 2-Cys peroxiredoxins in human parasites: several physiological roles for a potential chemotherapy target. Mol Biochem Parasitol 206(1-2):2–12. https://doi.org/10.1016/j.molbiopara.2016.03.005
Anschau V, Ferrer-Sueta G, Aleixo-Silva RL, Bannitz-Fernandes R, Tairum CA, Tonoli CCC, Murakami MT, de Oliveira MA, Netto LES (2020) Reduction of sulfenic acids by ascorbate in proteins, connecting thiol-dependent to alternative redox pathways. Free Radic Biol Med 156:207–216. https://doi.org/10.1016/j.freeradbiomed.2020.06.015
Baek WK, Lee HS, Oh MH, Koh MJ, Kim KS, Choi SH (2009) Identification of the Vibrio vulnificus ahpCl gene and its influence on survival under oxidative stress and virulence. J Microbiol 47(5):624–632. https://doi.org/10.1007/s12275-009-0130-x
Baker LM, Poole LB (2003) Catalytic mechanism of thiol peroxidase from Escherichia coli. Sulfenic acid formation and overoxidation of essential CYS61. J Biol Chem 278(11):9203–9211. https://doi.org/10.1074/jbc.M209888200
Baker LM, Raudonikiene A, Hoffman PS, Poole LB (2001) Essential thioredoxin-dependent peroxiredoxin system from Helicobacter pylori: genetic and kinetic characterization. J Bacteriol 183(6):1961–1973. https://doi.org/10.1128/JB.183.6.1961-1973.2001
Bannitz-Fernandes R, Aleixo-Silva R, Silva JP, Dodia C, Vazquez-Medina JP, Tao JQ, Fisher A, Netto L (2019) Non-mammalian Prdx6 enzymes (proteins with 1-Cys Prdx mechanism) display PLA(2) activity similar to the human orthologue. Antioxidants (Basel) 8(3). https://doi.org/10.3390/antiox8030052
Bottcher T, Sieber SA (2010) Showdomycin as a versatile chemical tool for the detection of pathogenesis-associated enzymes in bacteria. J Am Chem Soc 132(20):6964–6972. https://doi.org/10.1021/ja909150y
Brenot A, King KY, Caparon MG (2005) The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Mol Microbiol 55(1):221–234. https://doi.org/10.1111/j.1365-2958.2004.04370.x
Bryk R, Griffin P, Nathan C (2000) Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature 407(6801):211–215
Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C (2002) Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295(5557):1073–1077. https://doi.org/10.1126/science.1067798
Budde H, Flohe L (2003) Enzymes of the thiol-dependent hydroperoxide metabolism in pathogens as potential drug targets. Biofactors 17(1-4):83–92. https://doi.org/10.1002/biof.5520170109
Buranajitpakorn S, Piwkam A, Charoenlap N, Vattanaviboon P, Mongkolsuk S (2011) Genes for hydrogen peroxide detoxification and adaptation contribute to protection against heat shock in Xanthomonas campestris pv. campestris. FEMS Microbiol Lett 317(1):60–66. https://doi.org/10.1111/j.1574-6968.2011.02211.x
Castro H, Teixeira F, Romao S, Santos M, Cruz T, Florido M, Appelberg R, Oliveira P, Ferreira-da-Silva F, Tomas AM (2011) Leishmania mitochondrial peroxiredoxin plays a crucial peroxidase-unrelated role during infection: insight into its novel chaperone activity. PLoS Pathog 7(10):e1002325. https://doi.org/10.1371/journal.ppat.1002325
Castro H, Rocha MI, Silva R, Oliveira F, Gomes-Alves AG, Cruz T, Duarte M, Tomas AM (2020) Functional insight into the glycosomal peroxiredoxin of Leishmania. Acta Trop 201:105217. https://doi.org/10.1016/j.actatropica.2019.105217
Cha MK, Kim HK, Kim IH (1995) Thioredoxin-linked “thiol peroxidase” from periplasmic space of Escherichia coli. J Biol Chem 270(48):28635–28641. https://doi.org/10.1074/jbc.270.48.28635
Charoenlap N, Shen Z, McBee ME, Muthupalani S, Wogan GN, Fox JG, Schauer DB (2012) Alkyl hydroperoxide reductase is required for Helicobacter cinaedi intestinal colonization and survival under oxidative stress in BALB/c and BALB/c interleukin-10-/- mice. Infect Immun 80(3):921–928. https://doi.org/10.1128/IAI.05477-11
Chen H, Liu Y, Zhao C, Xiao D, Zhang J, Zhang F, Chen M, Wang H (2013) Comparative proteomics-based identification of genes associated with glycopeptide resistance in clinically derived heterogeneous vancomycin-intermediate Staphylococcus aureus strains. PLoS One 8(6):e66880. https://doi.org/10.1371/journal.pone.0066880
Cho C, Lee GW, Hong SH, Kaur S, Jung KW, Jung JH, Lim S, Chung BY, Lee SS (2019) Novel functions of peroxiredoxin Q from Deinococcus radiodurans R1 as a peroxidase and a molecular chaperone. FEBS Lett 593(2):219–229. https://doi.org/10.1002/1873-3468.13302
Choi W, Yoo YJ, Kim M, Shin D, Jeon HB, Choi W (2003) Identification of proteins highly expressed in the hyphae of Candida albicans by two-dimensional electrophoresis. Yeast 20(12):1053–1060. https://doi.org/10.1002/yea.1022
Comtois SL, Gidley MD, Kelly DJ (2003) Role of the thioredoxin system and the thiol-peroxidases Tpx and Bcp in mediating resistance to oxidative and nitrosative stress in Helicobacter pylori. Microbiology (Reading) 149(Pt 1):121–129. https://doi.org/10.1099/mic.0.25896-0
Condeles AL, Gomes F, de Oliveira MA, Soares Netto LE, Toledo Junior JC (2020) Thiol peroxidases as major regulators of intracellular levels of peroxynitrite in live Saccharomyces cerevisiae cells. Antioxidants (Basel) 9(5). https://doi.org/10.3390/antiox9050434
Copley SD, Novak WR, Babbitt PC (2004) Divergence of function in the thioredoxin fold suprafamily: evidence for evolution of peroxiredoxins from a thioredoxin-like ancestor. Biochemistry 43(44):13981–13995. https://doi.org/10.1021/bi048947r
Cosgrove K, Coutts G, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ (2007) Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J Bacteriol 189(3):1025–1035. https://doi.org/10.1128/JB.01524-06
Das UN (2018) Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: a review. J Adv Res 11:57–66. https://doi.org/10.1016/j.jare.2018.01.001
Davis PH, Zhang X, Guo J, Townsend RR, Stanley SL Jr (2006) Comparative proteomic analysis of two Entamoeba histolytica strains with different virulence phenotypes identifies peroxiredoxin as an important component of amoebic virulence. Mol Microbiol 61(6):1523–1532. https://doi.org/10.1111/j.1365-2958.2006.05344.x
de Godoy LM, Marchini FK, Pavoni DP, Rampazzo Rde C, Probst CM, Goldenberg S, Krieger MA (2012) Quantitative proteomics of Trypanosoma cruzi during metacyclogenesis. Proteomics 12(17):2694–2703. https://doi.org/10.1002/pmic.201200078
Dons LE, Mosa A, Rottenberg ME, Rosenkrantz JT, Kristensson K, Olsen JE (2014) Role of the Listeria monocytogenes 2-Cys peroxiredoxin homologue in protection against oxidative and nitrosative stress and in virulence. Pathog Dis 70(1):70–74. https://doi.org/10.1111/2049-632X.12081
Dwyer DJ, Belenky PA, Yang JH, MacDonald IC, Martell JD, Takahashi N, Chan CT, Lobritz MA, Braff D, Schwarz EG, Ye JD, Pati M, Vercruysse M, Ralifo PS, Allison KR, Khalil AS, Ting AY, Walker GC, Collins JJ (2014) Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci U S A 111(20):E2100–E2109. https://doi.org/10.1073/pnas.1401876111
Enany S, Yoshida Y, Yamamoto T (2014) Exploring extra-cellular proteins in methicillin susceptible and methicillin resistant Staphylococcus aureus by liquid chromatography-tandem mass spectrometry. World J Microbiol Biotechnol 30(4):1269–1283. https://doi.org/10.1007/s11274-013-1550-7
Estelle D, Laurence L, Marc O, Caroline C, Magali D, Marie-Laure F (2020) Linolenic fatty acid hydroperoxide acts as biocide on plant pathogenic bacteria: biophysical investigation of the mode of action. Bioorg Chem 100:103877. https://doi.org/10.1016/j.bioorg.2020.103877
Fereig RM, Kuroda Y, Terkawi MA, Mahmoud ME, Nishikawa Y (2017) Immunization with Toxoplasma gondii peroxiredoxin 1 induces protective immunity against toxoplasmosis in mice. PLoS One 12(4):e0176324. https://doi.org/10.1371/journal.pone.0176324
Fisher RA, Gollan B, Helaine S (2017) Persistent bacterial infections and persister cells. Nat Rev Microbiol 15(8):453–464. https://doi.org/10.1038/nrmicro.2017.42
Flohé L, Budde H, Bruns K, Castro H, Clos J, Hofmann B, Kansal-Kalavar S, Krumme D, Menge U, Plank-Schumacher K, Sztajer H, Wissing J, Wylegalla C, Hecht H-J (2002) Tryparedoxin peroxidase of Leishmania donovani: molecular cloning, heterologous expression, specificity, and catalytic mechanism. Arch Biochem Biophys 397(2):324–335
Francis KP, Taylor PD, Inchley CJ, Gallagher MP (1997) Identification of the ahp operon of Salmonella typhimurium as a macrophage-induced locus. J Bacteriol 179(12):4046–4048. https://doi.org/10.1128/jb.179.12.4046-4048.1997
Gautam P, Shankar J, Madan T, Sirdeshmukh R, Sundaram CS, Gade WN, Basir SF, Sarma PU (2008) Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to amphotericin B. Antimicrob Agents Chemother 52(12):4220–4227. https://doi.org/10.1128/AAC.01431-07
Gautam P, Mushahary D, Hassan W, Upadhyay SK, Madan T, Sirdeshmukh R, Sundaram CS, Sarma PU (2016) In-depth 2-DE reference map of Aspergillus fumigatus and its proteomic profiling on exposure to itraconazole. Med Mycol 54(5):524–536. https://doi.org/10.1093/mmy/myv122
Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O'Shea EK, Weissman JS (2003) Global analysis of protein expression in yeast. Nature 425(6959):737–741. https://doi.org/10.1038/nature02046
Gu S, Chen J, Dobos KM, Bradbury EM, Belisle JT, Chen X (2003) Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Mol Cell Proteomics 2(12):1284–1296. https://doi.org/10.1074/mcp.M300060-MCP200
Gutierrez-Escobedo G, Hernandez-Carreon O, Morales-Rojano B, Revuelta-Rodriguez B, Vazquez-Franco N, Castano I, De Las PA (2020) Candida glabrata peroxiredoxins, Tsa1 and Tsa2, and sulfiredoxin, Srx1, protect against oxidative damage and are necessary for virulence. Fungal Genet Biol 135:103287. https://doi.org/10.1016/j.fgb.2019.103287
Hajaj B, Yesilkaya H, Benisty R, David M, Andrew PW, Porat N (2012) Thiol peroxidase is an important component of Streptococcus pneumoniae in oxygenated environments. Infect Immun 80(12):4333–4343. https://doi.org/10.1128/IAI.00126-12
Hall A, Karplus PA, Poole LB (2009) Typical 2-Cys peroxiredoxins—structures, mechanisms and functions. FEBS J 276(9):2469–2477. https://doi.org/10.1111/j.1742-4658.2009.06985.x
Hall A, Parsonage D, Poole LB, Karplus PA (2010) Structural evidence that peroxiredoxin catalytic power is based on transition-state stabilization. J Mol Biol 402(1):194–209. https://doi.org/10.1016/j.jmb.2010.07.022
Hall A, Nelson K, Poole LB, Karplus PA (2011) Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid Redox Signal 15(3):795–815. https://doi.org/10.1089/ars.2010.3624
Halliwell B, Gutteridge JMC (2015) Free radicals in biology and medicine, 5th edn. Oxford University Press, Oxford
Haraldsen JD, Liu G, Botting CH, Walton JG, Storm J, Phalen TJ, Kwok LY, Soldati-Favre D, Heintz NH, Muller S, Westwood NJ, Ward GE (2009) Identification of conoidin a as a covalent inhibitor of peroxiredoxin II. Org Biomol Chem 7:3040–3048. https://doi.org/10.1039/B901735F
Heym B, Stavropoulos E, Honoré N, Domenech P, Saint-Joanis B, Wilson TM, Collins DM, Colston MJ, Cole ST (1997) Effects of overexpression of the alkyl hydroperoxide reductase AhpC on the virulence and isoniazid resistance of Mycobacterium tuberculosis. Infect Immun 65(4):1395–1401
Hicks LD, Raghavan R, Battisti JM, Minnick MF (2010) A DNA-binding peroxiredoxin of Coxiella burnetii is involved in countering oxidative stress during exponential-phase growth. J Bacteriol 192(8):2077–2084. https://doi.org/10.1128/JB.01324-09
Hillas PJ, del Alba FS, Oyarzabal J, Wilks A, Ortiz de Montellano PR (2000) The AhpC and AhpD antioxidant defense system of Mycobacterium tuberculosis. J Biol Chem 275(25):18801–18809
Hillmann F, Bagramyan K, Strassburger M, Heinekamp T, Hong TB, Bzymek KP, Williams JC, Brakhage AA, Kalkum M (2016) The crystal structure of peroxiredoxin Asp f3 provides mechanistic insight into oxidative stress resistance and virulence of Aspergillus fumigatus. Sci Rep 6:33396. https://doi.org/10.1038/srep33396
Horst SA, Jaeger T, Denkel LA, Rouf SF, Rhen M, Bange FC (2010) Thiol peroxidase protects Salmonella enterica from hydrogen peroxide stress in vitro and facilitates intracellular growth. J Bacteriol 192(11):2929–2932. https://doi.org/10.1128/JB.01652-09
Horta BB, de Oliveira MA, Discola KF, Cussiol JRR, Netto LES (2010) Structural and biochemical characterization of peroxiredoxin Qβ from Xylella fastidiosa. J Biol Chem 285(21):16051–16065
Hu Y, Coates AR (2009) Acute and persistent Mycobacterium tuberculosis infections depend on the thiol peroxidase TpX. PLoS One 4(4):e5150. https://doi.org/10.1371/journal.pone.0005150
Hu H, Tian M, Li P, Bao Y, Guan X, Lian Z, Yin Y, Ding C, Yu S (2019) Brucella infection regulates peroxiredoxin-5 protein expression to facilitate intracellular survival by reducing the production of nitric oxide and reactive oxygen species. Biochem Biophys Res Commun 516(1):82–88
Hugo M, Turell L, Manta B, Botti H, Monteiro G, Netto LES, Alvarez B, Radi R, Trujillo M (2009) Thiol and Sulfenic acid oxidation of AhpE, the one-cysteine Peroxiredoxin from : kinetics, acidity constants, and conformational dynamics. Biochemistry 48(40):9416–9426
Hugo M, Van Laer K, Reyes AM, Vertommen D, Messens J, Radi R, Trujillo M (2014) Mycothiol/mycoredoxin 1-dependent reduction of the peroxiredoxin AhpE from Mycobacterium tuberculosis. J Biol Chem 289(8):5228–5239. https://doi.org/10.1074/jbc.M113.510248
Jacobson FS, Morgan RW, Christman MF, Ames BN (1989) An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage. Purification and properties. J Biol Chem 264(3):1488–1496
Jaeger T, Flohe L (2006) The thiol-based redox networks of pathogens: unexploited targets in the search for new drugs. Biofactors 27(1-4):109–120. https://doi.org/10.1002/biof.5520270110
Jaeger T, Budde H, Flohé L, Menge U, Singh M, Trujillo M, Radi R (2004) Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis. Arch Biochem Biophys 423(1):182–191
Jain M, Munoz-Bodnar A, Zhang S, Gabriel DW (2018) A secreted ‘Candidatus Liberibacter asiaticus’ peroxiredoxin simultaneously suppresses both localized and systemic innate immune responses in planta. Mol Plant-Microbe Interact 31(12):1312–1322. https://doi.org/10.1094/MPMI-03-18-0068-R
Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH, Lee JR, Lee SS, Moon JC, Yun JW, Choi YO, Kim WY, Kang JS, Cheong GW, Yun DJ, Rhee SG, Cho MJ, Lee SY (2004) Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell 117(5):625–635. https://doi.org/10.1016/j.cell.2004.05.002
Jeong W, Cha M-K, Kim I-H (2000) Thioredoxin-dependent Hydroperoxide peroxidase activity of Bacterioferritin Comigratory protein (BCP) as a new member of the Thiol-specific antioxidant protein (TSA)/alkyl Hydroperoxide peroxidase C (AhpC) family. J Biol Chem 275(4):2924–2930
Johnson NA, Liu Y, Fletcher HM (2004) Alkyl hydroperoxide peroxidase subunit C (ahpC) protects against organic peroxides but does not affect the virulence of Porphyromonas gingivalis W83. Oral Microbiol Immunol 19(4):233–239. https://doi.org/10.1111/j.1399-302X.2004.00145.x
Kaihami GH, Almeida JR, Santos SS, Netto LE, Almeida SR, Baldini RL (2014) Involvement of a 1-Cys peroxiredoxin in bacterial virulence. PLoS Pathog 10(10):e1004442. https://doi.org/10.1371/journal.ppat.1004442
Kamariah N, Eisenhaber B, Eisenhaber F, Gruber G (2018) Molecular mechanism of the Escherichia coli AhpC in the function of a chaperone under heat-shock conditions. Sci Rep 8(1):14151. https://doi.org/10.1038/s41598-018-32527-7
Kaufmann SHE, Springer B, Master S, Sander P, Zahrt T, McFalone M, Song J, Papavinasasundaram KG, Colston MJ, Boettger E, Deretic V (2001) Silencing of oxidative stress response in : expression patterns of in virulent and avirulent strains and effect of inactivation. Infect Immun 69(10):5967–5973
Kaneva IN, Longworth J, Sudbery PE, Dickman MJ (2018) Quantitative proteomic analysis in Candida albicans using SILAC-based mass spectrometry. Proteomics 18(5-6):e1700278. https://doi.org/10.1002/pmic.201700278
Kim SJ, Woo JR, Hwang YS, Jeong DG, Shin DH, Kim K, Ryu SE (2003) The tetrameric structure of Haemophilus influenza hybrid Prx5 reveals interactions between electron donor and acceptor proteins. J Biol Chem 278(12):10790–10798. https://doi.org/10.1074/jbc.M209553200
Koch O, Jäger T, Heller K, Khandavalli PC, Pretzel J, Becker K, Flohé L, Selzer PM (2013) Identification of M. tuberculosis thioredoxin reductase inhibitors based on high-throughput docking using constraints. J Med Chem 56(12):4849–4859. https://doi.org/10.1021/jm3015734
König J, Fairlamb AH (2007) A comparative study of type I and type II tryparedoxin peroxidases in. FEBS J 274(21):5643–5658
Koshkin A, Zhou XT, Kraus CN, Brenner JM, Bandyopadhyay P, Kuntz ID, Barry CE 3rd, Ortiz de Montellano PR (2004) Inhibition of Mycobacterium tuberculosis AhpD, an element of the peroxiredoxin defense against oxidative stress. Antimicrob Agents Chemother 48(7):2424–2430. https://doi.org/10.1128/AAC.48.7.2424-2430.2004
Kumar B, Sharma D, Sharma P, Katoch VM, Venkatesan K, Bisht D (2013) Proteomic analysis of Mycobacterium tuberculosis isolates resistant to kanamycin and amikacin. J Proteome 94:68–77. https://doi.org/10.1016/j.jprot.2013.08.025
La Carbona S, Sauvageot N, Giard JC, Benachour A, Posteraro B, Auffray Y, Sanguinetti M, Hartke A (2007) Comparative study of the physiological roles of three peroxidases (NADH peroxidase, Alkyl hydroperoxide reductase and Thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol Microbiol 66(5):1148–1163. https://doi.org/10.1111/j.1365-2958.2007.05987.x
Lam PL, Wong RS, Lam KH, Hung LK, Wong MM, Yung LH, Ho YW, Wong WY, Hau DK, Gambari R, Chui CH (2020) The role of reactive oxygen species in the biological activity of antimicrobial agents: an updated mini review. Chem Biol Interact 320:109023. https://doi.org/10.1016/j.cbi.2020.109023
Leitsch D, Williams CF, Hrdy I (2018) Redox pathways as drug targets in microaerophilic parasites. Trends Parasitol 34(7):576–589. https://doi.org/10.1016/j.pt.2018.04.007
Li W, Zhang S, Wang X, Yu J, Li Z, Lin W, Lin X (2018) Systematically integrated metabonomic-proteomic studies of Escherichia coli under ciprofloxacin stress. J Proteome 179:61–70. https://doi.org/10.1016/j.jprot.2018.03.002
Lim JG, Bang YJ, Choi SH (2014) Characterization of the Vibrio vulnificus 1-Cys peroxiredoxin Prx3 and regulation of its expression by the Fe-S cluster regulator IscR in response to oxidative stress and iron starvation. J Biol Chem 289(52):36263–36274. https://doi.org/10.1074/jbc.M114.611020
Longo LVG, Breyer CA, Novaes GM, Gegembauer G, Leitao NP Jr, Octaviano CE, Toyama MH, de Oliveira MA, Puccia R (2020) The human pathogen Paracoccidioides brasiliensis has a unique 1-Cys peroxiredoxin that localizes both intracellularly and at the cell surface. Front Cell Infect Microbiol 10:394. https://doi.org/10.3389/fcimb.2020.00394
Lynch AS, Robertson GT (2008) Bacterial and fungal biofilm infections. Annu Rev Med 59:415–428. https://doi.org/10.1146/annurev.med.59.110106.132000
Marshall AC, Kidd SE, Lamont-Friedrich SJ, Arentz G, Hoffmann P, Coad BR, Bruning JB (2019) Structure, mechanism, and inhibition of Thioredoxin Reductase. Antimicrob Agents Chemother 63(3)
Mastronicola D, Falabella M, Testa F, Pucillo LP, Teixeira M, Sarti P, Saraiva LM, Giuffre A (2014) Functional characterization of peroxiredoxins from the human protozoan parasite Giardia intestinalis. PLoS Negl Trop Dis 8(1):e2631. https://doi.org/10.1371/journal.pntd.0002631
Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449(7164):819–826. https://doi.org/10.1038/nature06246
Mir AA, Park SY, Abu Sadat M, Kim S, Choi J, Jeon J, Lee YH (2015) Systematic characterization of the peroxidase gene family provides new insights into fungal pathogenicity in Magnaporthe oryzae. Sci Rep 5:11831. https://doi.org/10.1038/srep11831
Mishra Y, Hall M, Locmelis R, Nam K, Soderberg CAG, Storm P, Chaurasia N, Rai LC, Jansson S, Schroder WP, Sauer UH (2017) Active-site plasticity revealed in the asymmetric dimer of AnPrx6 the 1-Cys peroxiredoxin and molecular chaperone from Anabaena sp. PCC 7210. Sci Rep 7(1):17151. https://doi.org/10.1038/s41598-017-17044-3
Missall TA, Pusateri ME, Lodge JK (2004) Thiol peroxidase is critical for virulence and resistance to nitric oxide and peroxide in the fungal pathogen, Cryptococcus neoformans. Mol Microbiol 51(5):1447–1458. https://doi.org/10.1111/j.1365-2958.2004.03921.x
Monteiro G, Horta BB, Pimenta DC, Augusto O, Netto LE (2007) Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc Natl Acad Sci U S A 104(12):4886–4891. https://doi.org/10.1073/pnas.0700481104
Morais MAB, Giuseppe PO, Souza T, Castro H, Honorato RV, Oliveira PSL, Netto LES, Tomas AM, Murakami MT (2017) Calcium and magnesium ions modulate the oligomeric state and function of mitochondrial 2-Cys peroxiredoxins in Leishmania parasites. J Biol Chem 292(17):7023–7039. https://doi.org/10.1074/jbc.M116.762039
Munhoz DC, Netto LES (2004) Cytosolic Thioredoxin peroxidase I and II are important defenses of yeast against organic Hydroperoxide insult. J Biol Chem 279(34):35219–35227
Murphy TF, Kirkham C, Sethi S, Lesse AJ (2005) Expression of a peroxiredoxin-glutaredoxin by Haemophilus influenzae in biofilms and during human respiratory tract infection. FEMS Immunol Med Microbiol 44(1):81–89. https://doi.org/10.1016/j.femsim.2004.12.008
Nelson KJ, Knutson ST, Soito L, Klomsiri C, Poole LB, Fetrow JS (2011) Analysis of the peroxiredoxin family: using active-site structure and sequence information for global classification and residue analysis. Proteins 79(3):947–964. https://doi.org/10.1002/prot.22936
Netto LE, de Oliveira MA, Tairum CA, da Silva Neto JF (2016) Conferring specificity in redox pathways by enzymatic thiol/disulfide exchange reactions. Free Radic Res 50(2):206–245. https://doi.org/10.3109/10715762.2015.1120864
Nogoceke E, Gommel DU, Kiess M, Kalisz HM, Flohe L (1997) A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem 378(8):827–836. https://doi.org/10.1515/bchm.1997.378.8.827
Nurnberger T, Brunner F, Kemmerling B, Piater L (2004) Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev 198:249–266. https://doi.org/10.1111/j.0105-2896.2004.0119.x
Ogusucu R, Rettori D, Munhoz DC, Netto LES, Augusto O (2007) Reactions of yeast thioredoxin peroxidases I and II with hydrogen peroxide and peroxynitrite: rate constants by competitive kinetics. Free Radic Biol Med 42(3):326–334
Ogusucu R, Rettori D, Netto LE, Augusto O (2009) Superoxide dismutase 1-mediated production of ethanol- and DNA-derived radicals in yeasts challenged with hydrogen peroxide: molecular insights into the genome instability of peroxiredoxin-null strains. J Biol Chem 284(9):5546–5556. https://doi.org/10.1074/jbc.M805526200
Olczak AA, Seyler RW Jr, Olson JW, Maier RJ (2003) Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infect Immun 71(1):580–583. https://doi.org/10.1128/iai.71.1.580-583.2003
Oliveira MA, Discola KF, Alves SV, Medrano FJ, Guimaraes BG, Netto LE (2010) Insights into the specificity of thioredoxin reductase-thioredoxin interactions. A structural and functional investigation of the yeast thioredoxin system. Biochemistry 49(15):3317–3326. https://doi.org/10.1021/bi901962p
O'Riordan AA, Morales VA, Mulligan L, Faheem N, Windle HJ, Kelleher DP (2012) Alkyl hydroperoxide reductase: a candidate Helicobacter pylori vaccine. Vaccine 30(26):3876–3884. https://doi.org/10.1016/j.vaccine.2012.04.002
Parente MR, Forte E, Falabella M, Boneca IG, Teixeira M, Giuffre A, Saraiva LM (2017) Nitrosative stress defences of the enterohepatic pathogenic bacterium Helicobacter pullorum. Sci Rep 7(1):9909. https://doi.org/10.1038/s41598-017-10375-1
Park SG, Cha MK, Jeong W, Kim IH (2000) Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J Biol Chem 275(8):5723–5732. https://doi.org/10.1074/jbc.275.8.5723
Parsonage D, Youngblood DS, Sarma GN, Wood ZA, Andrew Karplus P, Poole LB (2005) Analysis of the link between enzymatic activity and Oligomeric state in AhpC, a bacterial Peroxiredoxin. Biochemistry 44(31):10583–10592
Parsonage D, Karplus PA, Poole LB (2008) Substrate specificity and redox potential of AhpC, a bacterial peroxiredoxin. Proc Natl Acad Sci U S A 105(24):8209–8214. https://doi.org/10.1073/pnas.0708308105
Pedrajas JR, Miranda-Vizuete A, Javanmardy N, Gustafsson JA, Spyrou G (2000) Mitochondria of Saccharomyces cerevisiae contain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J Biol Chem 275(21):16296–16301. https://doi.org/10.1074/jbc.275.21.16296
Pedrajas JR, McDonagh B, Hernandez-Torres F, Miranda-Vizuete A, Gonzalez-Ojeda R, Martinez-Galisteo E, Padilla CA, Barcena JA (2016) Glutathione is the resolving thiol for thioredoxin peroxidase activity of 1-Cys peroxiredoxin without being consumed during the catalytic cycle. Antioxid Redox Signal 24(3):115–128. https://doi.org/10.1089/ars.2015.6366
Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA (2015) Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci 40(8):435–445. https://doi.org/10.1016/j.tibs.2015.05.001
Piacenza L, Peluffo G, Alvarez MN, Kelly JM, Wilkinson SR, Radi R (2008) Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem J 410(2):359–368. https://doi.org/10.1042/BJ20071138
Piñeyro MD, Parodi-Talice A, Arcari T, Robello C (2008) Peroxiredoxins from Trypanosoma cruzi: virulence factors and drug targets for treatment of Chagas disease? Gene 408(1–2):45–50
Pineyro MD, Arcari T, Robello C, Radi R, Trujillo M (2011) Tryparedoxin peroxidases from Trypanosoma cruzi: high efficiency in the catalytic elimination of hydrogen peroxide and peroxynitrite. Arch Biochem Biophys 507(2):287–295. https://doi.org/10.1016/j.abb.2010.12.014
Poole LB, Ellis HR (1996) Flavin-dependent alkyl hydroperoxide reductase from Salmonella typhimurium. 1. Purification and enzymatic activities of overexpressed AhpF and AhpC proteins. Biochemistry 35(1):56–64. https://doi.org/10.1021/bi951887s
Poole LB, Nelson KJ (2016) Distribution and features of the six classes of peroxiredoxins. Mol Cell 39(1):53–59. https://doi.org/10.14348/molcells.2016.2330
Poole LB, Hall A, Nelson KJ (2011) Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol Chapter 7:Unit7 9. https://doi.org/10.1002/0471140856.tx0709s49
Prolo C, Alvarez MN, Radi R (2014) Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. Biofactors 40(2):215–225. https://doi.org/10.1002/biof.1150
Radi R (2018) Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc Natl Acad Sci U S A 115(23):5839–5848. https://doi.org/10.1073/pnas.1804932115
Repolês BM, Machado CR, Florentino PT (2020) DNA lesions and repair in trypanosomatids infection. Genet Mol Biol 43(1):e20190163
Reyes AM, Hugo M, Trostchansky A, Capece L, Radi R, Trujillo M (2011) Oxidizing substrate specificity of Mycobacterium tuberculosis alkyl hydroperoxide reductase E: kinetics and mechanisms of oxidation and overoxidation. Free Radic Biol Med 51(2):464–473. https://doi.org/10.1016/j.freeradbiomed.2011.04.023
Reyes AM, Vazquez DS, Zeida A, Hugo M, Pineyro MD, De Armas MI, Estrin D, Radi R, Santos J, Trujillo M (2016) PrxQ B from Mycobacterium tuberculosis is a monomeric, thioredoxin-dependent and highly efficient fatty acid hydroperoxide reductase. Free Radic Biol Med 101:249–260. https://doi.org/10.1016/j.freeradbiomed.2016.10.005
Rhee SG, Kil IS (2017) Multiple functions and regulation of mammalian peroxiredoxins. Annu Rev Biochem 86:749–775. https://doi.org/10.1146/annurev-biochem-060815-014431
Richard D, Bartfai R, Volz J, Ralph SA, Muller S, Stunnenberg HG, Cowman AF (2011) A genome-wide chromatin-associated nuclear peroxiredoxin from the malaria parasite Plasmodium falciparum. J Biol Chem 286(13):11746–11755. https://doi.org/10.1074/jbc.M110.198499
Rintiswati N, Wibawa T, Asmara W, Soebono H (2011) Effect of oxidative stress on AhpC activity and virulence in katG Ser315 Thr Mycobacterium tuberculosis mutant. Indonesian J Biotechnol 16(2):100–110
Rocha ER, Smith CJ (1999) Role of the alkyl hydroperoxide reductase (ahpCF) gene in oxidative stress defense of the obligate Anaerobe Bacteroides fragilis. J Bacteriol 181(18):5701–5710. https://doi.org/10.1128/JB.181.18.5701-5710.1999
Rocha MC, de Godoy KF, Bannitz-Fernandes R, Fabri J, Barbosa MMF, de Castro PA, Almeida F, Goldman GH, da Cunha AF, Netto LES, de Oliveira MA, Malavazi I (2018) Analyses of the three 1-Cys peroxiredoxins from Aspergillus fumigatus reveal that cytosolic Prx1 is central to H2O2 metabolism and virulence. Sci Rep 8(1):12314. https://doi.org/10.1038/s41598-018-30108-2
Rodrigues AM, Kubitschek-Barreira PH, Pinheiro BG, Teixeira-Ferreira A, Hahn RC, de Camargo ZP (2020) Immunoproteomic analysis reveals novel candidate antigens for the diagnosis of paracoccidioidomycosis due to Paracoccidioides lutzii. J Fungi (Basel) 6(4). https://doi.org/10.3390/jof6040357
Saccoccia F, Di Micco P, Boumis G, Brunori M, Koutris I, Miele AE, Morea V, Sriratana P, Williams DL, Bellelli A, Angelucci F (2012) Moonlighting by different stressors: crystal structure of the chaperone species of a 2-Cys peroxiredoxin. Structure 20(3):429–439. https://doi.org/10.1016/j.str.2012.01.004
Seaver LC, Imlay JA (2001) Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol 183(24):7173–7181. https://doi.org/10.1128/JB.183.24.7173-7181.2001
Sherman DR, Mdluli K, Hickey MJ, Arain TM, Morris SL, Barry CE 3rd, Stover CK (1996) Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science 272(5268):1641–1643. https://doi.org/10.1126/science.272.5268.1641
Shishodia SK, Tiwari S, Shankar J (2019) Resistance mechanism and proteins in Aspergillus species against antifungal agents. Mycology 10(3):151–165. https://doi.org/10.1080/21501203.2019.1574927
Silva KSE, Neto BRS, Zambuzzi-Carvalho PF, de Oliveira CM, Pires LB, Kato L, Bailao AM, Parente-Rocha JA, Hernandez O, Ochoa JG, Soares MC, Pereira M (2018) Response of Paracoccidioides lutzii to the antifungal camphene thiosemicarbazide determined by proteomic analysis. Future Microbiol 13:1473–1496. https://doi.org/10.2217/fmb-2018-0176
Skrzypek MS, Binkley J, Binkley G, Miyasato SR, Simison M, Sherlock G (2017) The Candida Genome Database (CGD): incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res 45(D1):D592–D596. https://doi.org/10.1093/nar/gkw924
Souza Goebel C, de Mattos Oliveira F, Severo LC (2013) Saccharomyces cerevisiae infections. Rev Iberoam Micol 30(3):205–208. https://doi.org/10.1016/j.riam.2013.03.001
Staerck C, Gastebois A, Vandeputte P, Calenda A, Larcher G, Gillmann L, Papon N, Bouchara JP, Fleury MJJ (2017) Microbial antioxidant defense enzymes. Microb Pathog 110:56–65. https://doi.org/10.1016/j.micpath.2017.06.015
Staudacher V, Djuika CF, Koduka J, Schlossarek S, Kopp J, Büchler M, Lanzer M, Deponte M (2015) Plasmodium falciparum antioxidant protein reveals a novel mechanism for balancing turnover and inactivation of peroxiredoxins. Free Radic Biol Med 85:228–236
Storz G, Christman MF, Sies H, Ames BN (1987) Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc Natl Acad Sci U S A 84(24):8917–8921. https://doi.org/10.1073/pnas.84.24.8917
Tairum CA, Santos MC, Breyer CA, de Oliveira ALP, Cabrera VIM, Toledo-Silva G, Mori GM, Toyama MH, Netto LES, de Oliveira MA (2021) Effects of Serine or Threonine in the active site of typical 2-Cys Prx on hyperoxidation susceptibility and on chaperone activity. Antioxidants (Basel). https://doi.org/10.3390/antiox10071032
Tairum CA, Santos MC, Breyer CA, Geyer RR, Nieves CJ, Portillo-Ledesma S, Ferrer-Sueta G, Toledo JC Jr, Toyama MH, Augusto O, Netto LE, de Oliveira MA (2016) Catalytic Thr or Ser residue modulates structural switches in 2-Cys peroxiredoxin by distinct mechanisms. Sci Rep 6:33133. https://doi.org/10.1038/srep33133
Taylor PD, Inchley CJ, Gallagher MP (1998) The Salmonella typhimurium AhpC polypeptide is not essential for virulence in BALB/c mice but is recognized as an antigen during infection. Infect Immun 66(7):3208–3217. https://doi.org/10.1128/IAI.66.7.3208-3217.1998
Teixeira F, Castro H, Cruz T, Tse E, Koldewey P, Southworth DR, Tomas AM, Jakob U (2015) Mitochondrial peroxiredoxin functions as crucial chaperone reservoir in Leishmania infantum. Proc Natl Acad Sci U S A 112(7):E616–E624. https://doi.org/10.1073/pnas.1419682112
Trujillo M, Budde H, Piñeyro MD, Stehr M, Robello C, Flohé L, Radi R (2004) Trypanosoma brucei and Trypanosoma cruzi Tryparedoxin peroxidases catalytically detoxify Peroxynitrite via oxidation of fast reacting Thiols. J Biol Chem 279(33):34175–34182
Tunes LG, Morato RE, Garcia A, Schmitz V, Steindel M, Corrêa-Junior JD, Dos Santos HF, Frézard F, de Almeida MV, Silva H, Moretti NS, de Barros ALB, do Monte-Neto RL (2020) Preclinical gold complexes as oral drug candidates to treat leishmaniasis are potent trypanothione reductase inhibitors. ACS Infect Dis 6(5):1121–1139. https://doi.org/10.1021/acsinfecdis.9b00505
Urban C, Xiong X, Sohn K, Schroppel K, Brunner H, Rupp S (2005) The moonlighting protein Tsa1p is implicated in oxidative stress response and in cell wall biogenesis in Candida albicans. Mol Microbiol 57(5):1318–1341. https://doi.org/10.1111/j.1365-2958.2005.04771.x
Usui M, Masuda-Suganuma H, Fukumoto S, Angeles JM, Hakimi H, Inoue N, Kawazu S (2015) Effect of thioredoxin peroxidase-1 gene disruption on the liver stages of the rodent malaria parasite Plasmodium berghei. Parasitol Int 64(3):290–294. https://doi.org/10.1016/j.parint.2014.09.013
Vallejo MC, Nakayasu ES, Matsuo AL, Sobreira TJ, Longo LV, Ganiko L, Almeida IC, Puccia R (2012) Vesicle and vesicle-free extracellular proteome of Paracoccidioides brasiliensis: comparative analysis with other pathogenic fungi. J Proteome Res 11(3):1676–1685. https://doi.org/10.1021/pr200872s
Vediyappan G, Rossignol T, d'Enfert C (2010) Interaction of Candida albicans biofilms with antifungals: transcriptional response and binding of antifungals to beta-glucans. Antimicrob Agents Chemother 54(5):2096–2111. https://doi.org/10.1128/AAC.01638-09
Vranakis I, De Bock PJ, Papadioti A, Tselentis Y, Gevaert K, Tsiotis G, Psaroulaki A (2012) Quantitative proteome profiling of C. burnetii under tetracycline stress conditions. PLoS One 7(3):e33599. https://doi.org/10.1371/journal.pone.0033599
Wang G, Olczak AA, Walton JP, Maier RJ (2005) Contribution of the Helicobacter pylori thiol peroxidase bacterioferritin comigratory protein to oxidative stress resistance and host colonization. Infect Immun 73(1):378–384. https://doi.org/10.1128/IAI.73.1.378-384.2005
Wilson T, de Lisle GW, Marcinkeviciene JA, Blanchardand JS, Collins DM (1998) Antisense RNA to ahpC, an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology 144(10):2687–2695
Winterbourn CC, Hampton MB (2008) Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45(5):549–561. https://doi.org/10.1016/j.freeradbiomed.2008.05.004
Winterbourn CC, Kettle AJ, Hampton MB (2016) Reactive oxygen species and neutrophil function. Annu Rev Biochem 85:765–792. https://doi.org/10.1146/annurev-biochem-060815-014442
Wood ZA, Poole LB, Karplus PA (2003) Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300(5619):650–653. https://doi.org/10.1126/science.1080405
Yague-Capilla M, Garcia-Caballero D, Aguilar-Pereyra F, Castillo-Acosta VM, Ruiz-Perez LM, Vidal AE, Gonzalez-Pacanowska D (2019) Base excision repair plays an important role in the protection against nitric oxide- and in vivo-induced DNA damage in Trypanosoma brucei. Free Radic Biol Med 131:59–71. https://doi.org/10.1016/j.freeradbiomed.2018.11.025
Yu S, Girotto S, Lee C, Magliozzo RS (2003) Reduced affinity for isoniazid in the S315T mutant of Mycobacterium tuberculosis KatG is a key factor in antibiotic resistance. J Biol Chem 278(17):14769–14775. https://doi.org/10.1074/jbc.M300326200
Zhu X, Tu ZJ, Coussens PM, Kapur V, Janagama H, Naser S, Sreevatsan S (2008) Transcriptional analysis of diverse strains Mycobacterium avium subspecies paratuberculosis in primary bovine monocyte derived macrophages. Microbes Infect 10(12-13):1274–1282. https://doi.org/10.1016/j.micinf.2008.07.025
Zygmunt MS, Hagius SD, Walker JV, Elzer PH (2006) Identification of Brucella melitensis 16M genes required for bacterial survival in the caprine host. Microbes Infect 8(14-15):2849–2854. https://doi.org/10.1016/j.micinf.2006.09.002
Funding
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The authors M.A.O., C.A.T., L.E.S.N, R.L.A.S., A.L.P.O., V.I.M.C., and. M.C.S. are members of the CEPID REDOXOMA from FAPESP (grant number 2013/07937-8). This work was also supported by grants from FAPESP to M.A.O. (2017/19942-7). C.A.T., A.L.P.O, R.L.A.S., V.I.M.C., M.C.S., and C.A.B. are recipients of fellowships from FAPESP (16/15849-0; 19/04054-4; 20/00845-4; 20/02868-1; 17/06263-4; and 11/13500-6, respectively).
Author information
Authors and Affiliations
Contributions
All authors wrote, revised, edited, and approved the final version of the paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira, M.A., Tairum, C.A., Netto, L.E.S. et al. Relevance of peroxiredoxins in pathogenic microorganisms. Appl Microbiol Biotechnol 105, 5701–5717 (2021). https://doi.org/10.1007/s00253-021-11360-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11360-5