Abstract
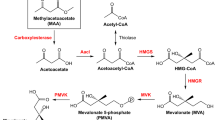
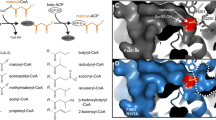
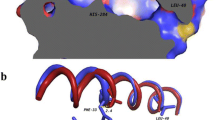
Plant waxes are interesting substitutes of fossil-derived compounds; however, their limited sources and narrow structural diversity prompted the development of microbial platforms to produce esters with novel chemical structures and properties. One successful strategy was the heterologous expression of the mycocerosic polyketide synthase-based biosynthetic pathway (MAS-PKS, PapA5 and FadD28 enzymes) from Mycobacterium tuberculosis in Escherichia coli. This recombinant strain has the ability to produce a broad spectrum of multimethyl-branched long-chain esters (MBE) with novel chemical structures and high oxidation stability. However, one limitation of this microbial platform was the low yields obtained for MBE derived of short-chain alcohols. In an attempt to improve the titers of the short-chain alcohol-derived MBE, we focused on the PapA5 acyltransferase—enzyme that catalyzes the ester formation reaction. Specific amino acid residues located in the two-substrate recognition channels of this enzyme were identified, rationally mutated, and the corresponding mutants characterized both in vivo and in vitro. The phenylalanine located at 331 position in PapA5 (F331) was found to be a key residue that when substituted by other bulky and aromatic or bulky and polar amino acid residues (F331W, F331Y or F331H), gave rise to PapA5 mutants with improved bioconversion efficiency; showing in average, 2.5 higher yields of short-chain alcohol-derived MBE compared with the wild-type enzyme. Furthermore, two alternative pathways for synthetizing ethanol were engineered into the MBE producer microorganism, allowing de novo production of ethanol-derived MBE at levels comparable with those obtained by the external supply of this alcohol.
Key points
• Mutation in channel 2 changes PapA5 acyltransferase bioconversion efficiency.
• Improved production of short-chain alcohol derived multimethyl-branched esters.
• Establishing ethanologenic pathways for de novo production of ethanol derived MBE.
• Characterization of a novel phenylethanol-derived MBE.






Similar content being viewed by others
References
Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–90. https://doi.org/10.1038/nature06450
Barney BM, Mann RL, Ohlert JM (2013) Identification of a residue affecting fatty alcohol selectivity in wax ester synthase. Appl Environ Microbiol 79:396–399. https://doi.org/10.1128/AEM.02523-12
Barney BM, Ohlert JM, Timler JG, Lijewski AM (2015) Altering small and medium alcohol selectivity in the wax ester synthase. Appl Microbiol Biotechnol 99:9675–9684. https://doi.org/10.1007/s00253-015-6783-y
Berka K, Hanak O, Sehnal D, Banas P, Navratilova V, Jaiswal D, Varekova SR, Koca J, Otyepka M (2012) MOLE online 2 . 0 : interactive web-based analysis of biomacromolecular channels. Nucleic Acids Res 40:222–227. https://doi.org/10.1093/nar/gks363
Bisht RPS, Sivasankaran GA, Bhatia VK (1993) Additive properties of jojoba oil for lubricating oil formulations. Wear 161:193–197. https://doi.org/10.1016/0043-1648(93)90469-3
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Buglino J, Onwueme K, Ferreras JA, Quadri LEN, Lima CD (2004) Crystal structure of PapA5, a phthiocerol dimycocerosyl transferase from Mycobacterium tuberculosis. J Biol Chem 279:30634–30642
Conway EJ (1962) Microdifussion analysis and volumetric error. Crosby Lockwood & Son Ltd, London
Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. https://doi.org/10.1128/jb.179.16.5094-5103.1997
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Hatzios SK, Schelle MW, Holsclaw CM, Behrens CR, Botyanszki Z, Lin FL, Carlson BL, Kumar P, Leary JA, Bertozzi CR (2009) PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis. J Biol Chem 284:12745–12751. https://doi.org/10.1074/jbc.M809088200
Holland-Staley CA, Lee K, Clark DP, Cunningham PR (2000) Aerobic activity of Escherichia coli alcohol dehydrogenase is determined by a single amino acid. J Bacteriol 182:6049–6054. https://doi.org/10.1128/JB.182.21.6049-6054.2000
Ingram LO, Conway T, Clark DP, Sewell GW, Preston JF (1987) Genetic engineering of ethanol production in Escherichia coli. Appl Environ Microbiol 53:2420–2425
Janßen HJ, Steinbüchel A (2014) Fatty acid synthesis in Escherichia coli and its applications towards the production of fatty acid based biofuels. Biotechnol Biofuels 7:1–26. https://doi.org/10.1186/1754-6834-7-7
Jogl G, Tong L (2003) Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell 112:113–122
Kalscheuer R, Stölting T, Steinbüchel A (2006) Microdiesel: Escherichia coli engineered for fuel production. Microbiology 152:2529–2536. https://doi.org/10.1099/mic.0.29028-0
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358. https://doi.org/10.1126/science.1193990
Kumar P, Schelle MW, Jain M, Lin FL, Petzold CJ, Leavell MD, Leary JA, Cox JS, Bertozzi CR (2007) PapA1 and PapA2 are acyltransferases essential for the biosynthesis of the Mycobacterium tuberculosis virulence factor Sulfolipid-1. Proc Natl Acad Sci U S A 104:11221–11226. https://doi.org/10.1073/pnas.0611649104
Leslie AGW (1990) Refined crystal structure of type III chloramphenicol acetyltransferase at 1 . 75 A resolution. J Mol Biol 213:167–186
Lewicka AJ, Lyczakowski JJ, Blackhurst G, Pashkuleva C, Rothschild-Mancinelli K, Tautvaišas D, Thornton H, Villanueva H, Xiao W, Sikas J, Horsfall L, Elfick A, French C (2014) Fusion of pyruvate decarboxylase and alcohol dehydrogenase increases ethanol production in Escherichia coli. ACS Synth Biol 3:976–978. https://doi.org/10.1021/sb500020g
Longo MA, Sanromán MA (2006) Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol Biotechnol 44:335–353. https://doi.org/10.1201/9780429441837-15
Marella ER, Holkenbrink C, Siewers V, Borodina I (2018) Engineering microbial fatty acid metabolism for biofuels and biochemicals. Curr Opin Biotechnol 50:39–46. https://doi.org/10.1016/j.copbio.2017.10.002
Menendez-Bravo S, Comba S, Sabatini M, Arabolaza A, Gramajo H (2014) Expanding the chemical diversity of natural esters by engineering a polyketide-derived pathway into Escherichia coli. Metab Eng 24:97–106. https://doi.org/10.1016/j.ymben.2014.05.002
Menendez-Bravo S, Roulet J, Sabatini M, Comba S, Dunn R, Gramajo H, Arabolaza A (2016) High cell density production of multimethyl-branched long-chain esters in Escherichia coli and determination of their physicochemical properties. Biotechnol Biofuels 9:215. https://doi.org/10.1186/s13068-016-0631-x
Menendez-Bravo S, Comba S, Gramajo H, Arabolaza A (2017) Metabolic engineering of microorganisms for the production of structurally diverse esters. Appl Microbiol Biotechnol 101:3043–3053. https://doi.org/10.1007/s00253-017-8179-7
Molnos J, Gardiner R, Dale GE, Lange R (2003) A continuous coupled enzyme assay for bacterial malonyl-CoA:acyl carrier protein transacylase (FabD). Anal Biochem 319:171–176. https://doi.org/10.1016/S0003-2697(03)00327-0
Murli S, Kennedy J, Dayem LC, Carney JR, Kealey JT (2003) Metabolic engineering of Escherichia coli for improved 6-deoxyerythronolide B production. J Ind Microbiol Biotechnol 30:500–509. https://doi.org/10.1007/s10295-003-0073-x
Ngo HL, Dunn RO, Sharma B, Foglia TA (2011) Synthesis and physical properties of isostearic acids and their esters. Eur J Lipid Sci Technol 113:180–188. https://doi.org/10.1002/ejlt.201000335
Onwueme KC, Ferreras JA, Buglino J, Lima CD, Quadri LEN (2004) Mycobacterial polyketide-associated proteins are acyltransferases: proof of principle with Mycobacterium tuberculosis PapA5. Proc Natl Acad Sci U S A 101:4608–4613. https://doi.org/10.1073/pnas.0306928101
Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C (2001) Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291:1790–1792. https://doi.org/10.1126/science.1058092
Rodriguez GM, Tashiro Y, Atsumi S (2014) Expanding ester biosynthesis in Escherichia coli. Nat Chem Biol 10:259–265. https://doi.org/10.1016/j.physbeh.2017.03.040
Rombouts Y, Alibaud L, Carrère-Kremer S, Maes E, Tokarski C, Elass E, Kremer L, Guérardel Y (2011) Fatty acyl chains of Mycobacterium marinum lipooligosaccharides: structure, localization and acylation by PapA4 (MMAR-2343) protein. J Biol Chem 286:33678–33688. https://doi.org/10.1074/jbc.M111.273920
Röttig A, Steinbüchel A (2013) Acyltransferases in bacteria. Microbiol Mol Biol Rev 77:277–321. https://doi.org/10.1128/MMBR.00010-13
Steen EJ, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463:559–563. https://doi.org/10.1038/nature08721
Stöveken T, Kalscheuer R, Malkus U, Reichelt R, Steinbüchel A (2005) The wax ester synthase/acyl coenzyme a:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: characterization of a novel type of acyltransferase. J Bacteriol 187:1369–1376. https://doi.org/10.1128/JB.187.4.1369
Touchette MH, Bommineni GR, Delle Bovi RJ, Gadbery JE, Nicora CD, Shukla AK, Kyle JE, Metz TO, Martin DW, Sampson NS, Miller WT, Tonge PJ, Seeliger JC (2015) Diacyltransferase activity and chain length specificity of Mycobacterium tuberculosis PapA5 in the synthesis of alkyl β-diol lipids. Biochemistry 54:5457–5468. https://doi.org/10.1021/acs.biochem.5b00455
Trivedi OA, Arora P, Vats A, Ansari MZ, Tickoo R, Sridharan V, Mohanty D, Gokhale RS (2005) Dissecting the mechanism and assembly of a complex virulence mycobacterial lipid. Mol Cell 17:631–643. https://doi.org/10.1016/j.molcel.2005.02.009
Wolfmeier U, Schmidt H, Heinrichs FL, Michalczyk G, Payer W, Dietsche W, Boehlike K, Hohner G, Wildgruber J (2000) Waxes. In: Ullmann’s Encyclopedia of Industrial Chemistry, vol 39, pp 1–204. https://doi.org/10.1002/14356007.a28
Xu P, Gu Q, Wang W, Wong L, Bower AGW, Collins CH, Koffas MAG (2013) Modular optimization of multi-gene pathways for fatty acids production in E. coli. Nat Commun 4:1409. https://doi.org/10.1038/ncomms2425
Xu P, Li L, Zhang F, Stephanopoulos G, Koffas M (2014) Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci U S A 111:11299–11304. https://doi.org/10.1073/pnas.1406401111
Xu Y, Wang S, Hu Q, Gao S, Ma X, Zhang W, Shen Y, Chen F, Lai L, Pei J (2018) CavityPlus : a web server for protein cavity detection with pharmacophore modelling , allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res 46:374–379. https://doi.org/10.1093/nar/gky380
Yan Q, Pfleger BF (2020) Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab Eng 58:35–46. https://doi.org/10.1016/j.ymben.2019.04.009
Youngquist JT, Schumacher MH, Rose JP, Raines TC, Politz MC, Copeland MF, Pfleger BF (2013) Production of medium chain length fatty alcohols from glucose in Escherichia coli. Metab Eng 20:177–186. https://doi.org/10.1016/j.ymben.2013.10.006
Zhou YJ, Buijs NA, Zhu Z, Qin J, Siewers V, Nielsen J (2016) Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat Commun 7:11709. https://doi.org/10.1038/ncomms11709
Acknowledgments
We are grateful to Dr. Christopher R. French and Marco Valenzuela for kindly providing pSB1K2 plasmids. We thank Guillermo Marcuzzi for technical assistance in the GC-MS experiments. MS, AA, and HG are members of the Research Career; VC is a member of the FBIOyF Faculty; VG is a doctoral fellow of CONICET; and JR and JL were doctoral fellows of CONICET.
Funding
This work was supported by PID-2013-0042 grant to HG and PICT-2017-2184 to AA.
Author information
Authors and Affiliations
Contributions
AA and HG conceived and designed the research. JR, VG, AA, and HG designed the experiments and analyzed the data. JR and VG performed all the experiments. JL collaborated with the structural analysis. VC and MS helped in specific experimental methods. AA and HG wrote the manuscript. All the authors read and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 730 kb).
Rights and permissions
About this article
Cite this article
Roulet, J., Galván, V., Lara, J. et al. Modification of PapA5 acyltransferase substrate selectivity for optimization of short-chain alcohol-derived multimethyl-branched ester production in Escherichia coli. Appl Microbiol Biotechnol 104, 8705–8718 (2020). https://doi.org/10.1007/s00253-020-10872-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10872-w