Abstract
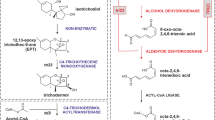
Trichothecenes are sesquiterpene toxins produced by diverse but relatively few fungal species in at least three classes of Ascomycetes: Dothideomycetes, Eurotiomycetes, and Sordariomycetes. Approximately 200 structurally distinct trichothecene analogs have been described, but a given fungal species typically produces only a small subset of analogs. All trichothecenes share a core structure consisting of a four-ring nucleus known as 12,13-epoxytrichothec-9-ene. This structure can be substituted at various positions with hydroxyl, acyl, or keto groups to give rise to the diversity of trichothecene structures that has been described. Over the last 30 years, the genetic and biochemical pathways required for trichothecene biosynthesis in several species of the fungi Fusarium and Trichoderma have been elucidated. In addition, phylogenetic and functional analyses of trichothecene biosynthetic (TRI) genes from fungi in multiple genera have provided insights into how acquisition, loss, and changes in functions of TRI genes have given rise to the diversity of trichothecene structures. These analyses also suggest both divergence and convergence of TRI gene function during the evolutionary history of trichothecene biosynthesis. What has driven trichothecene structural diversification remains an unanswered question. However, insight into the role of trichothecenes in plant pathogenesis of Fusarium species and into plant glucosyltransferases that detoxify the toxins by glycosylating them point to a possible driver. Because the glucosyltransferases can have substrate specificity, changes in trichothecene structures produced by a fungus could allow it to evade detoxification by the plant enzymes. Thus, it is possible that advantages conferred by evading detoxification have contributed to trichothecene structural diversification.
Key Points
• TRI genes have evolved by diverse processes: loss, acquisition and changes in function.
• Some TRI genes have acquired the same function by convergent evolution.
• Some other TRI genes have evolved divergently to have different functions.
• Some TRI genes were acquired or resulted from diversification in function of other genes.
• Substrate specificity of plant glucosyltransferases could drive trichothecene diversity.









Similar content being viewed by others
References
Alexander NJ, McCormick SP, Ziegenhorn SL (1999) Phytotoxicity of selected trichothecenes using Chlamydomonas reinhardtii as a model system. Nat Toxins 7:265–269
Alexander NJ, Proctor RH, McCormick SP (2009) Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev 28:198–215
Alexander NJ, McCormick SP, Waalwijk C, van der Lee T, Proctor RH (2011) The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet Biol 48:485–495
Brown DW, McCormick SP, Alexander NJ, Proctor RH, Desjardins AE (2002) Inactivation of a cytochrome P-450 is a determinant of trichothecene diversity in Fusarium species. Fungal Genet Biol 36:224–233
Brown DW, Proctor RH, Dyer RB, Plattner RD (2003) Characterization of a Fusarium 2-gene cluster involved in trichothecene C-8 modification. J Agric Food Chem 51:7936–7944
Brown DW, Dyer RB, McCormick SP, Kendra DF, Plattner RD (2004) Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal Genet Biol 41:454–462
Cardoza RE, Malmierca MG, Hermosa MR, Alexander NJ, McCormick SP, Proctor RH, Tijerino AM, Rumbero A, Monte E, Gutiérrez S (2011) Identification of loci and functional characterization of trichothecene biosynthesis genes in filamentous fungi of the genus Trichoderma. Appl Environ Microbiol 77:4867–4877. https://doi.org/10.1128/AEM.00595-11
Cardoza RE, McCormick SP, Malmierca MG, Olivera ER, Alexander NJ, Monte E, Gutiérrez S (2015) Effects of trichothecene production on the plant defense response and fungal physiology: overexpression of the Trichoderma arundinaceum tri4 gene in T. harzianum. Appl Environ Microbiol 81:6355–6366
Cardoza RE, McCormick SP, Lindo L, Kim H-S, Olivera ER, Nelson DR, Proctor RH, Gutiérrez S (2019) A cytochrome P450 monooxygenase gene required for biosynthesis of the trichothecene toxin harzianum A in Trichoderma. Appl Microbiol Biotechnol 103:8087–8103. https://doi.org/10.1007/s00253-019-10047-2
Carrasco L, Barbacid M, Vazquez D (1973) The trichodermin group of antibiotics, inhibitors of peptide bond formation by eukaryotic ribosomes. Biochim Biophys Acta 312:368–376
Cole R, Jarvis BB, Schweikert MA (2003) Handbook of secondary fungal metabolites volume III. Academic Press, San Diego
Cundliffe E, Davies JE (1977) Inhibition of initiation, elongation, and termination of eukaryotic protein synthesis by trichothecene fungal toxins. Antimicrob Agents Chemother 11:491–499
Cundliffe E, Cannon M, Davies J (1974) Mechanism of inhibition of eukaryotic protein synthesis by trichothecene fungal toxins. Antimicrob Agents Chemother 11:491–499
Desjardins AE, Proctor RH, Bai G, McCormick SP, Shaner G, Beuchley G, Hohn TM (1996) Reduced virulence of trichothecene-non-producing mutants of Gibberella zeae in wheat field tests. Mol Plant-Microbe Interact 9:1996–1023
Desjardins AE, McCormick SP, Appell M (2007) Structure-activity relationships of trichothecene toxins in an Arabidopsis thaliana leaf assay. J Agric Food Chem 55:6487–6492. https://doi.org/10.1021/jf0709193
Helliwell CA, Poole A, Peacock J, Dennis ES (1999) Arabidopsis ent-kaurene oxidase catalyzes three steps of gibberellin biosynthesis. Plant Physiol 119:507–510
Hoffmeister D, Keller NP (2007) Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep 24:393–416
Hohn TM, Beremand P (1989) Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene 79:131–138
Jarvis BB (1991) Macrocyclic trichothecenes. In: Sharma RP, Salunkhe DK (eds) Mycotoxins and Phytoalexins. CRC Press, Inc., Boca Raton, pp 361–421
Keller NP, Turner G, Bennett JW (2005) Fungal secondary metabolism- from biochemistry to genomics. Nat Rev Microbiol 3:937–947. https://doi.org/10.1038/nrmicro1286
Kelly A, Proctor RH, Belzile F, Chulze SN, Clear RM, Cowger G, Elmer W, Lee T, Obanor F, Waalwijk C, Ward T (2016) The geographic distribution and complex evolutionary history of the NX-2 trichothecene chemotype from Fusarium graminearum. Fungal Genet Biol 95:39–48. https://doi.org/10.1016/j.fgb.2016.08.003
Khatibi PA, Newmister SA, Rayment I, McCormick SP, Alexander NJ, Schmale DG (2011) Bioprospecting for trichothecene 3-O-acetyltransferases in the fungal genus Fusarium yields functional enzymes with different abilities to modify the mycotoxin deoxynivalenol. Appl Environ Microbiol 77:1162–1170
Kikuchi H, Miyagawa Y, Sahashi Y, Inatomi S, Haganuma A, Nakahata N, Oshima Y (2004) Novel spirocyclic trichothecanes, spirotenuipesine A and B, isolated from entomopathogenic fungus, Paecilomyces tenuipes. J Organomet Chem 69:352–356
Kimura M, Kaneko I, Komiyama M, Takatsuki A, Koshino H, Yoneyama K, Yamaguchi I (1998a) Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. J Biol Chem 273:1654–1661
Kimura M, Matsumoto G, Shingu Y, Yoneyama K, Yamaguchi I (1998b) The mystery of the trichothecene 3-O-acetyltransferase gene analysis of the region around Tri101 and characterization of its homologue from Fusarium sporotrichioides. FEBS Lett 435:163–168
Kimura M, Tokai T, Matsumoto G, Fujimura M, Hamamoto H, Yoneyama K, Shibata T, Yamaguchi I (2003) Trichothecene nonproducer Gibberella species have both functional and nonfunctional 3-O-actyltransferase genes. Genetics 163:677–684
Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M (2007) Molecular and genetic studies of Fusarium trichothecene biosynthesis: pathways, genes, and evolution. Biosci Biotechnol Biochem 71:2105–2123
Lee T, Han YK, Kim KH, Yun SH, Lee YW (2002) Tri13 and Tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl Environ Microbiol 68:2148–2154
Li XZ, Zhu C, de Lange CF, Zhou T, He J, Yu H, Gong J, Young JC (2011) Efficacy of detoxification of deoxynivalenol-contaminated corn by Bacillus sp. LS100 in reducing the adverse effects of the mycotoxin on swine growth performance. Food Addit Contam Part A 28:894–901
Li X, Michlmayr H, Scheweiger W, Malachova A, Shin S, Huang Y, Dong Y, Wiesenberger G, McCormick S, Lemmens M, Fruhmann P, Hametner C, Berthiller F, Adam G, Muehlbauer GJ (2017) A barley UDP-glucosyltransferase inactivates nivalenol and provides Fusarium head blight resistance in transgenic wheat. J Exp Bot 68:2187–2197. https://doi.org/10.1093/jxb/erx109
Lindo L, McCormick SP, Cardoza RE, Brown DW, Kim H-S, Alexander NJ, Proctor RH, Gutiérrez S (2018) Effect of deletion of a trichothecene toxin regulatory gene on the secondary metabolism transcriptome of the saprotrophic fungus Trichoderma arundinaceum. Fungal Genet Biol 119:29–46. https://doi.org/10.1016/j.fgb.2018.08.002
Lindo L, McCormick SP, Cardoza RE, Busman M, Alexander NJ, Proctor RH, Gutiérrez S (2019a) Requirement of two acyltransferases for 4-O-acylation during biosynthesis of harzianum A, an antifungal trichothecene produced by Trichoderma arundinaceum. J Agric Food Chem 67:723–734. https://doi.org/10.1021/acs.jafc.8b05564
Lindo L, McCormick SP, Cardoza RE, Kim H-S, Brown DW, Alexander NJ, Proctor RH, Gutiérrez S (2019b) Role of Trichoderma arundinaceum tri10 in regulation of terpene biosynthetic genes and in control of metabolic flux. Fungal Genet Biol 122:31–46
Malmierca MG, Cardoza RE, Alexander NJ, McCormick SP, Collado IG, Hermosa R, Monte E, Gutiérrez S (2013) Relevance of trichothecenes in fungal physiology: disruption of tri5 in Trichoderma arundinaceum. Fungal Genet Biol 53:22–33
Marcet-Houben M, Gabaldon T (2019) Evolutionary and functional patterns of shared gene neighbourhood in fungi. Nat Microbiol 4:2383–2392. https://doi.org/10.1038/s41564-019-0552-0
McCormick SP, Alexander NJ (2002) Fusarium Tri8 encodes a trichothecene C-3 esterase. Appl Environ Microbiol 68:2959–2964
McCormick SP, Alexander NJ (2007) Myrothecium roridum Tri4 encodes a multifunctional oxygenase required for three oxygenation steps. Can J Microbiol 53:572–579
McCormick SP, Hohn TM, Desjardins AE (1996) Isolation and characterization of Tri3, a gene encoding 15-O-acetyltransferase from Fusarium sporotrichioides. Appl Environ Microbiol 62:353–359
McCormick SP, Harris LJ, Alexander NJ, Ouellet T, Saparno A, Allard S, Desjardins AE (2004) Tri1 in Fusarium graminearum encodes a P450 oxygenase. Appl Environ Microbiol 70:2044–2451
McCormick SP, Alexander NJ, Proctor RH (2006) Fusarium Tri4 encodes a multifunctional oxygenase required for trichothecene biosynthesis. Can J Microbiol 52:636–642
McCormick SP, Stanley AM, Stover NA, Alexander NJ (2011) Trichothecenes: from simple to complex mycotoxins. Toxins 3:802–814. https://doi.org/10.3390/toxins3070802
McLaughlin JE, Bin-Umer MA, Tortora A, Mendeze N, McCormick S, Tumer NE (2009) A genome-wide screen in Saccharomyces cerevisiae reveals a critical role for the mitochondria in the toxicity of a trichothecene mycotoxin. Proc Natl Acad Sci U S A 106:21883–21888
McLean M (1996) The phytotoxicity of Fusarium metabolites: an update since 1989. Mycopathologia 133:163–179
Meek IB, Peplow AW, Ake C Jr, Phillips TD, Beremand MN (2003) Tri1 encodes the cytochrome P450 monooxygenase for C-8 hydroxylation during trichothecene biosynthesis in Fusarium sporotrichioides and resides upstream of another new Tri gene. Appl Environ Microbiol 69:1607–1613
Michlmayr H, Varga E, Malachová A, Fruhmann P, Piatkowska M, Hametner C, Sofrová J, Jaunecker G, Häubl G, Lemmens M, Berthiller F, Adam G (2018) UDP-Glucosyltransferases from rice, Brachypodium, and barley: substrate specificities and synthesis of type A and B trichothecene-3-O-β-D-glucosides. Toxins 10:111. https://doi.org/10.3390/toxins10030111
Middlebrook JL, Leatherman DL (1989) Specific associations of T-2 toxin with mammalian cells. Biochem Pharmacol 38:3093–3102
Mondol MA, Surovy MZ, Islam MT, Schuffler A, Laatsch H (2015) Macrocyclic trichothecenes from Myrothecium roridum strain M10 with motility inhibitory and zoosporicidal activities against Phytophthora nicotianae. J Agric Food Chem 63:8777–8786. https://doi.org/10.1021/acs.jafc.5b02366
Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2014) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Pace JG, Watts MR, Canterbury WJ (1988) T-2 mycotoxin inhibits mitochondrial protein synthesis. Toxicon 26:77–85
Peplow AW, Meek IB, Wiles MC, Phillips TD, Beremand MN (2003) Tri16 is required for esterification of position C-8 during trichothecene mycotoxin production by Fusarium sporotrichioides. Appl Environ Microbiol 69:5935–5940
Pestka JJ (2008) Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit Contam 22:1128–1140
Pitt JI, Lange L, Lacey AE, Vuong D, Midgley DJ, Greenfield P, Bradbury MI, Lacey E, Busk PK, Pilgaard B, Chooi H, Piggott AM (2017) Aspergillus hancockii sp. nov., a biosynthetically talented fungus endemic to southeastern Australian soils. PLoS One 12(4):e0170254. https://doi.org/10.1371/journal.pone.0170254
Proctor RH, Hohn TM, McCormick SP (1995) Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol Plant-Microbe Interact 61:1923–1930
Proctor RH, Hohn TM, McCormick SP (1997) Restoration of wild-type virulence to Tri5 disruption mutants of Gibberella zeae via gene reversion and mutant complementation. Microbiology 143:2583–2591
Proctor RH, McCormick SP, Alexander NJ, Desjardins AE (2009) Evidence that a secondary metabolic biosynthetic gene cluster has grown by gene relocation during evolution of the filamentous fungus Fusarium. Mol Microbiol 74:1128–1142. https://doi.org/10.1111/j.1365-2958.2009.06927.x
Proctor RH, McCormick SP, Kim H-S, Cardoza RE, Stanley AM, Lindo L, Kelly A, Brown DW, Lee T, Vaughan MM, Alexander NJ, Busman M, Gutiérrez S (2018) Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLoS Pathog 14:e1006946. https://doi.org/10.1371/journal.ppat.1006946
Riley RT, Norred WP (1996) Mechanisms of mycotoxicity. In: Howard DH, Miller JD (eds) The Mycota VI: human and animal relationships. Springer-Verlag, New York, pp 193–211
Ro DK, Arimura GI, Lau SYW, Piers E, Bohlmann J (2005) Loblolly pine abietadienol/abietadienal oxidase PtAO (CYP702B1) is a multifunctional, multisubstrate cytochrome P450 monooxygenase. Proc Natl Acad Sci U S A 102:8060–8065. https://doi.org/10.1073/pnas.0500825102
Rosso M, Maier M, Bertoni M (2000) Macrocyclic trichothecene production by the fungus epibiont of Baccharis coridifolia. Molecules 5:345–347
Salichos L, Stamatakis A, Rokas A (2014) Novel information theory-based measures for quantifying incongruence among phylogenetic trees. Mol Biol Evol 31(5):1261–1271. https://doi.org/10.1093/molbev/msu061
Semeiks J, Borek D, Otwinowski Z, Grishin NV (2014) Comparative genome sequencing reveals chemotype-specific gene clusters in the toxigenic black mold Stachybotrys. BMC Genomics 15:590. https://doi.org/10.1186/1471-2164-15-590
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Straus DC (2009) Molds, mycotoxins, and sick building syndrome. Toxicol Ind Health 25:617–635. https://doi.org/10.1177/0748233709348287
Sundstøl Eriksen G, Pettersson H, Lundh T (2004) Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem Toxicol 42:619–624
Suneja SK, Wagle DS, Ram GC (1989) Effect of oral administration of T-2 toxin on glutathione shuttle enzymes, microsomal reductases and lipid peroxidation in rat liver. Toxicon 27:995–1001
Surup F, Medjedović A, Szczygielski M, Schroers H-J, Stadler M (2014) Production of trichothecenes by the apple sooty blotch fungus Microcyclospora tardicrescens. J Agric Food Chem 62:3525–3530
Tamm C, Breitenstein W (1980) The biosynthesis of trichothecene Mycotoxins. In: Steyn P (ed) The biosynthesis of mycotoxins: a study in secondary metabolism. Academic Press, New York, pp 69–104
Tijerino A, Cardoza RE, Moraga J, Malmierca MG, Vicente F, Aleu J, Collado IG, Gutiérrez S, Monte E, Hermosa R (2011) Overexpression of the trichodiene synthase gene tri5 increases trichodermin production and antimicrobial activity in Trichoderma brevicompactum. Fungal Genet Biol 48:285–296. https://doi.org/10.1016/j.fgb.2010.11.012
Tokai T, Fujimura M, Inoue H, Aoki T, Ohta K, Shibata T, Yamaguchi I, Kimura M (2005) Concordant evolution of trichothecene 3-O-acetyltransferase and an rDNA species phylogeny of trichothecene-producing and non-producing fusaria and other ascomycetous fungi. Microbiology 151(2):509–519
Tokai T, Takahashi-Ando N, Izawa M, Kamakura T, Yoshida M, Fujimura M, Kimura M (2008) 4-O-acetylation and 3-oacetylation of trichothecenes by trichothecene 15-O-acetyltransferase encoded by Fusarium Tri3. Biosci Biotechnol Biochem 72:2485–2489. https://doi.org/10.1271/bbb.80501
Trapp SC, Hohn TM, McCormick SP, Jarvis BB (1998) Characterization of the macrocyclic trichothecene gene cluster in Myrothecium roridum. Mol Gen Genet 257:421–432
Tudzynski B (2005) Gibberellin biosynthesis in fungi: genes, enzymes, evolution, and impact on biotechnology. Appl Microbiol Biotechnol 66:597–611. https://doi.org/10.1007/s00253-004-1805-1
Ueno Y (1977) Mode of action of trichothecenes. Ann Nutr Aliment 31:885–900
Ueno Y (1984) Toxicological features of T-2 toxin and related trichothecenes. Fundam Appl Toxicol 4:S124–S132
Ueno Y (1985) The toxicology of mycotoxins. Crit Rev Toxicol 14:99–132
Ueno Y, Matsumoto H (1975) Inactivation of some thiol-enzymes by trichothecene mycotoxins from Fusarium species. Chem Pharm Bull 23:2439–2442
Varga E, Wiesenberger G, Hametner C, Ward TJ, Dong Y, Schöfbeck D, McCormick SP, Broz K, Stückler R, Schuhmacher R, Krska R, Kistler HC, Berthiller F, Adam G (2015) New tricks of an old enemy: isolates of Fusarium graminearum produce a type a trichothecene mycotoxin. Environ Microbiol 17:2588–2600. https://doi.org/10.1111/1462-2920.12718
Venkatasubbaiah P, Sutton TB, Chilton WS (1995) The structure and biological properties of secondary metabolites produced by Peltaster fructicola, a fungus associated with apple sooty blotch disease. Plant Dis 79:1157–1160
Villani A, Proctor RH, Kim H-S, Brown DW, Logrieco AF, Amatulli MT, Moretti A, Susca A (2019) Variation in secondary metabolite production potential in the Fusarium incarnatum-equiseti species complex revealed by comparative analysis of 13 genomes. BMC Genomics 20:314. https://doi.org/10.1186/s12864-019-5567-7
Wannemacher RW, Winer SL (1977) Trichothecene mycotoxins. In: Sidell RR, Takafuji ET, Franz DR (eds) Medical Aspects of Chemical and Biological Warfare. Office of the Surgeon General at TMM Publications, Washington, DC, USA, pp 655–676
Wetterhorn KM, Gabardi K, Michlmayr H, Malachova A, Busman M, McCormick SP, Berthiller F, Adam G, Rayment I (2017) Determinants and expansion of specificity in a Trichothecene UDP-Glucosyltransferase from Oryza sativa. Biochemistry 56:6585–6596. https://doi.org/10.1021/acs.biochem.7b01007
Ye W, Liu T, Zhu M, Zhang W, Li H, Huang Z, Li S (2017) De novo transcriptome analysis of plant pathogenic fungus Myrothecium roridum and identification of genes associated with trichothecene mycotoxin biosynthesis. Int J Mol Sci 18:497
Availability of data and material
Not applicable.
Funding
This work was supported by the Spanish Ministry of Science, Innovation and Universities (MCINN-RTI2018-099600-B-I00 to SG) and the Food Safety National Program of the Agriculture Research Service, US Department of Agriculture (SPM and RHP).
Author information
Authors and Affiliations
Contributions
RHP, SPM, and SG conceived the study and wrote the article. RHP and SG formatted the article to its final version. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Authors declare their consent to participate in this article.
Consent for publication
Authors declare their consent to publish this article.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
†Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Proctor, R.H., McCormick, S.P. & Gutiérrez, S. Genetic bases for variation in structure and biological activity of trichothecene toxins produced by diverse fungi. Appl Microbiol Biotechnol 104, 5185–5199 (2020). https://doi.org/10.1007/s00253-020-10612-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10612-0