Abstract
Dengue virus (DENV) is a vector-borne human pathogen that usually causes dengue fever; however, sometime it leads to deadly complications such as dengue with warning signs (DWS+) and severe dengue (SD). Several studies have shown that fusion (Fu) and bc loop of DENV envelope domain II are highly conserved and consist some of the most dominant antigenic epitopes. Therefore, in this study, Fu and bc loops were joined together to develop a short recombinant protein as an alternative of whole DENV envelope protein, and its immunogenic potential as fusion peptide was estimated. For de novo designing of the antigen, Fu and bc peptides were linked with an optimised linker so that the three dimensional conformation was maintained as it is in DENV envelope protein. The redesigned Fubc protein was expressed in E. coli and purified. Subsequently, structural integrity of the purified protein was verified by CD spectroscopy. To characterise immune responses against recombinant Fubc protein, BALB/c mice were subcutaneously injected with emulsified antigen preparation. It was observed by ELISA that Fubc fusion protein elicited higher serum IgG antibody response either in the presence or in absence of Freund’s adjuvant in comparison to the immune response of Fu and bc peptides separately. Furthermore, the binding of Fubc protein with mice antisera was validated by SPR analysis. These results suggest that Fu and bc epitope-based recombinant fusion protein could be a potential candidate towards the development of the effective subunit vaccine against DENV.
Similar content being viewed by others
Introduction
In the recent decades, dengue has emerged as one of the world’s most dominant tropical diseases (Guzman et al. 2016) with around 390 million (95% credible interval 284 to 528 million) cases recorded per year, of which only 96 million (95% credible interval 67 to 136 million) cases were manifested clinically (Giraldo-García and Castaño-Osorio, 2019; Bhatt et al. 2013). According to World Health Organization (WHO), an estimate of 3.9 billion people in 128 countries are living in areas with a high risk of dengue infection (Brady et al. 2012). The recently estimated annual 390 million dengue cases reveals that the dengue disease burden has tripled as compared to previous predictions of 50 to 100 million reported cases without dengue warning signs. Nonetheless, 250,000 to 500,000 patients were hospitalised due to dengue with warning signs (DWS+) and severe dengue (SD), and the total annual cost of dengue burden was estimated globally around US$ 8.9 billion (Giraldo-García and Castaño-Osorio, 2019; Guzman et al. 2016). In spite of having paucity of effective vaccines and drugs to expel dengue comprehensively, still it’s enduring a big challenge to human (Martina et al. 2009). Therefore, next-generation vaccine strategies such as inactivated purified virus, DNA or protein-based subunit vaccines, and their fusion chimeras are now going under investigation and some of them even under clinical trials (Coller et al. 2011; Danko et al. 2011; Liu et al. 2015).
Recently, Dengvaxia (CYD-TDV), a tetravalent live attenuated vaccine has been approved for use in some of the highly dengue endemic areas where the sero-prevalence is higher than 70% (Guy et al. 2015; Pang et al. 2018). According to WHO, it has shown significant efficacy and acceptable safety profile during clinical trials in seropositive individuals; however, it carries a risk of severe dengue infection in seronegative individuals, and also failed to confer subsequent protection in DENV-2 positive people in areas with less exposure of dengue (Arredondo-García et al. 2018; Durbin et al. 2011; Guy et al. 2011). Several other vaccine candidates were also formulated based on live-attenuated dengue viruses (e.g. TV003, TV005) and inactivated purified virus (e.g. DPIV), of which some have already completed clinical trials and some are currently going under phase II to III clinical trials (Whitehead et al. 2017; Diaz et al. 2018). On the other hand, DNA or protein-based alternative subunit vaccines (e.g. TVDV, DEN-80) and dengue-flavivirus chimeras (e.g. EDIII-p64k, 80E-STF2, EDIII-HbsAg) are going either under preclinical study or phase I clinical test (Govindarajan et al. 2015; Danko et al. 2018; Castaño-Osorio et al., 2019). Although, still most of them are seemed to be slow immunity booster and require a strong adjuvant and longer immunisation to achieve full protection against each of the dengue serotype, which all make them ill-suited for universal vaccine licence (Castaño-Osorio et al., 2019).
The dengue envelope (E) protein is composed of three ecto-domains, a membrane-proximal stem and a transmembrane anchor (Klein et al. 2013). Various crystal structures have shown that the ecto-domains are arranged into three antiparallel dimer on virus surface in a icosahedral symmetry, where ecto-domain I (EI) is located at the centre holding domain II (EDII) and III (EDIII) in two opposite sites in each of the monomer of a dimeric unit (Fibriansah et al. 2015; Kuhn et al. 2002; Modis et al. 2004). Most of the protective antibodies against dengue virus are identified to domain III, and initially were thought to be a potential subunit vaccine target (Murphy and Whitehead 2011; Sukupolvi-Petty et al. 2010). Although, recombinant EDIII domain injected by plasmid DNA or purified from bacterial expression systems was found to be poorly immunogenic, and has shown low protective efficacy in animal model (Guzman et al. 2010). On the other hand, EDII has been reported as the dimerisation domain and consists of the most conserved fusion (Fu) and bc loop (Li et al. 2019; Zhang et al. 2004). Several studies have also shown that the conserved Fu loop is highly immunogenic (Lai et al. 2008; Cherrier et al. 2009; Smith et al. 2013), and induces cross-reactive antibodies, of which some are cross-talk with adjacent bc loop (Gallichotte et al., 2015; Cherrier et al. 2009). Moreover, during endocytosis, both of the loops are found to involve in tertiary conformation of membrane fusion (Nayak et al. 2009; Sukupolvi-Petty et al. 2010). Therefore, it has been anticipated that both of the loops might have integrated role for boosting cross-neutralizing immunity.
In this study, we aim to develop an alternative vaccine by combining Fu and bc loop together in a single ORF for production of a fusion antigen. Although, the recombinant antigen in isolation tends to be poorly immunogenic in vivo; the use of potent immunomodulating compounds, fusion partner or suitable delivery systems improve specific immune response (Higgins et al. 2007; Garçon et al. 2011). Herein, we have expressed the recombinant Fubc antigenic protein, optimised its large-scale purification protocol and finally evaluated its protein-specific immune response in BALB/c mice. Prior to the animal challenge, its secondary structure was checked by CD spectroscopy, and binding specificity was cross-checked with a characterised anti-fusion loop scFv antibody (Rathore et al. 2019).
Materials and methods
Animals
All of the male and female BALB/c mice were obtained from National Institute of Nutrition, Telangana, Hyderabad, India and were maintained in standard light: dark (12:12) cycle with the supplement of adequate standard food, and water was provided from ad libitum. All of the animals were acclimated and randomly distributed into different experimental groups. Furthermore, all the in vivo experiments were performed in accordance with the committee for the purpose of control and supervision on experiments on animals (CPCSEA) guidelines and were approved by the South Asian University Institutional Animal Ethics Committee (IAEC) that was responsible for the care and use of laboratory animals.
Construction and cloning of Fubc gene
The DNA sequence of Fubc gene was retrieved from fusion (Fu) and bc loop of DENV serotype 2 envelope protein deposited in the protein data bank (PDB: 1OAN). In order to construct stable and immunogenic protein, highly conserved fusion (Fu) and bc loops and their neighbouring residues (amino acid residues 62 to 122) were selected by using antigen variability analyser (AVANA), pBLAST and multiple sequence alignment tool of NCBI. For convenient expression in bacteria, all of the oligos were codon optimised for E. coli, using codon optimisation tool of Integrated DNA Technology. Complete Fubc gene was constructed by assembly PCR reactions using four 60 nucleotide long overlapping oligonucleotides. For cloning into pET28a expression vector, two unique restriction sites, EcoRI and XhoI, were also incorporated at the 5′ and 3′ ends respectively during the final amplification of Fubc full-length gene using forward and reverse primers. Initially, the full-length Fubc gene was cloned into a TA cloning vector using InsTAclone kit (Thermo Scientific). Positive clones were screened using X-gal blue-white screening method and digested with EcoRI and XhoI restriction enzymes. The digested Fubc gene was further sub-cloned into a pET28a expression vector. The recombinant plasmid (Fubc + pET28a) was transformed into E. coli XL-10 GOLD for cloning, and subsequently into E. coli BL-21 Rosetta (DE3) for protein expression.
Expression of recombinant Fubc protein
The E. coli BL-21 (Rosetta) cells carrying Fubc gene in pET28a vector were grown overnight at 37 °C in 10 ml LB broth (Luria-Bertani medium) containing 50 μg/ml kanamycin (Sigma, USA). Overnight grown 1 ml primary culture was used to inoculate kanamycin containing 100 ml secondary culture and was further incubated at 37 °C. The incubated secondary culture was induced by 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) when its OD600 reached at around 0.5, and the culture was grown for another 4 h at 37 °C. The cells were harvested by centrifugation at 4000 rpm for 10 min. The resulting cell pellet was re-suspended in lysis buffer containing 50 mM Tris-HCl pH 8.0, 1 mM CaCl2, with 0.5% Triton X-100, Lysozyme 0.1 mg/ml, 1 mM EDTA and 1 mM PMSF. Then, the resuspended cells were kept on a rocker for an hour at room temperature, and sonication was done using 30% amplitude for five times 30 s on/off pulse. The lysed sample was separated into supernatant and pellet by centrifugation at 10,000 rpm for 10 min at 4 °C. Finally, Fubc expression level in supernatant and pellet were checked on 15% SDS-PAGE.
Solubilisation and purification of Fubc protein
A major fraction of the Fubc protein was observed in pellet after a number of efforts made to recover it in a soluble form. Then, it was decided to recover soluble protein from pellet fraction. The resulting pellet protein was washed 4 to 5 times with TE 50/20 (50 mM Tris pH 8.0 and 20 mM EDTA 20) buffer to remove impurities and extra salts. The remaining pellet was re-solubilised using mild denaturing agent such as 4.0 M urea and 5% n-propanol along with PBS buffer (pH 8.1) (Sarker et al. 2019) and was centrifuged at 20,000 rpm for 10 min. The soluble protein fraction was initially purified by using Ni-NTA agarose beads and was confirmed by western blot using anti-His antibody. However, the purity and yield were not sufficient. Therefore, soluble protein fraction was subjected to gel filtration in Superose 6 10/300 column by using fast protein liquid chromatography (FPLC) for large-scale good quality protein production. The column was pre-equilibrated with PBS (50 mM phosphate buffer pH 7.4 and 150 mM NaCl), and the protein sample was eluted with the same PBS buffer. 0.5 ml protein sample was injected in each run by using 0.5 ml loop at 0.4 ml/min flow rate. The elution profile of injected protein was followed by monitoring UV absorbance at 280 nm on the AKTA FPLC system with U9-L UV monitor. Different peaks greater than 20 mAU were collected in fraction collector and checked using a 15% SDS PAGE. Multiple runs of FPLC were carried out, and the fraction containing Fubc protein was pooled together. Finally, Fubc was concentrated and desalted by using 0.5 ml Millipore-Amicone filter with a cut-off of 3 kDa. Concentration of the purified Fubc was also measured by using BCA protein assay kit.
CD spectroscopy of recombinant Fubc protein
In order to assure the proper folding of purified (> 95%) Fubc protein, secondary structure was analysed by CD spectroscopy at far UV wavelength ranging from 180 to 260 nm. To achieve the best conformational reading, CD spectrum was obtained at the different protein and buffer concentrations, because the CD spectrum of a protein needs to be adequately intense for interpreting the data as the intensity of a CD spectrum directly relies on the protein and buffer concentration (Kelly et al. 2005; Miles and Wallace 2006). The CD spectra of PBS buffer and Fubc protein samples at 0.1 mg/ml and 0.2 mg/ml concentrations (diluted in 50 mM and 10 mM PBS buffer, pH 7.4) were recorded at far UV spectra ranging from 200 to 280 nm with a step size of 1 nm to bandwidth 1 nm. The measurement was performed at room temperature (22 °C), and the UV spectra were recorded for each sample with five scans. The baseline CD spectrum of the buffer was deducted from the spectrum containing the protein to yield the actual Fubc protein CD spectrum. The mean residue ellipticity [θ] mrw at wavelength λ was quoted in units of degree cm2/dmol, and was calculated as [θ] mrw, λ = MRW × θλ/10 × d × c, where θ is the observed ellipticity (degrees) at wavelength λ, d is the path length (cm) and c is the concentration (g/ml). MRW is the mean residual weight for the peptide bond which is given as MRW = M/(N − 1); where M is the molecular mass of the polypeptide chain (in Da), and N is the number of amino acids in the chain; the number of peptide bonds is N − 1.
Adjuvants and preparation of Fubc immunogens
Complete and incomplete Freund’s adjuvant (Sigma, USA) was used for primary (day 0) and booster immunisation (days 7, 14 and 21) respectively. To prepare a primary dose of the immunogen, complete Freund’s adjuvant (FA) was mixed with equal volume of purified Fubc (25 μg) in PBS and emulsified by vigorous vortex. Similarly, three booster doses of immunogen were prepared with an equal volume of Fubc protein (25 μg) and incomplete Freund’s adjuvant. All of the immunogens were prepared according to the protocol “Immunization of Mice” by Maira-Litrán, 2017. Finally, emulsified Fubc immunogens were checked by observing stable droplet on the water surface.
Experimental design for mice immunisation
Healthy BALB/c mice (10 weeks old, male and female) were randomly distributed into four different groups. Each group was immunised with different antigen preparations. Out of the four, two groups were immunised with Fubc antigen: one group was injected with Fubc protein with adjuvant and other was with Fubc protein without adjuvant. Rest of the two groups were immunised with Fu and bc peptide: one group was injected with Fu and Bc peptides with adjuvant, and other was Fu and bc peptide without adjuvant. Each of the groups was also subdivided into male and female sets, and with each set, one adjuvant control was used replacing adjuvant with PBS buffer. The mice of each group were subcutaneously immunised with emulsified antigen preparations, first dose (day 0) with CFA and three subsequent booster doses (at days 7, 14 and 21) with IFA (according to protocol Maira-Litrán 2017). After 5 days of the final booster dose, blood samples were collected by retro-orbital cavity, and all the antisera isolated from blood were stored at − 80 °C for further analysis.
ELISA to check murine IgG response against Fubc protein
Recombinant Fubc protein-specific IgG was measured by indirect ELISA. For this, 96 well ELISA plates were coated by incubating overnight at 4 °C with Fubc protein (1 μg/well) in 50 mM sodium carbonate-bicarbonate buffer pH 9.5. Blocking was done for 2 h with 2% BSA in PBST (PBS plus 0.05% Tween 20). Serum collected from immunised and control mice were incubated for 1 h at 37 °C after making 7 double dilutions in PBST starting from 1/500 μl. Subsequently, the ELISA plate was washed once with PBST, and 100 μl of anti-mouse HRP-conjugated secondary antibody (1:1000 dilution in PBST) was added and incubated for 30 min at room temperature. Then, the plate was washed three more times with PBST, and the binding reaction was developed by adding 200 μl/well OPD substrate (prepared in a phosphate-citrate buffer, 0.05 M, pH − 5.0 plus 0.03% H2O2). The reaction was stopped by adding 50 μl of 2.5 M H2SO4 just after observing an optimum colour, and the absorbance was measured at 492-nm wavelength using BioTek Synergy HT microplate reader (Winooski, VT). Mean absorbance was plotted against anti-sera dilutions, and two-way ANOVA was performed to find a statistically significant difference between two groups. Comparison of the immune response in different groups of antisera was made by converting each ELISA curve to linear equations (y = mx + c), and computing serum dilutions for a fixed ELISA absorbance. The data are expressed as mean ± standard deviation (SD). p values of < 0.05 were considered statistically significant.
Validation of Fubc immune response by SPR
To validate the immune response generated by recombinant Fubc, collected mice anti-sera were allowed for SPR interaction experiment with a Autolab ESPRIT instrument. Similar to ELISA, here also recombinant Fubc protein of DENV envelope was immobilised on a gold plate of SPR device. For coupling of Fubc protein on gold disc, 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC) and N-hydroxysuccinimide (NHS) were applied for activation. NHS activates the carboxymethyl groups by creating a highly reactive succinimide ester on the disc surface, which reacts with amine and other nucleophilic groups on proteins that subsequently help to bind the target protein on activated disc surface. Ethanolamine was added to block the remaining activated carboxymethyl group. For the qualitative assay, all of the serum samples (diluted in running buffer) were applied at a flow rate of 20 μl/min over 2 min. In between injections, the surface of the sensor chip was regenerated by injecting 2 M NaCl at the same flow rate for 15 s. In addition, surface coupling was done by using buffer (150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20 and 10 mM HEPES-NaOH) at pH 7.4, and the buffer containing 50 mM phosphate and 150 mM NaCl at pH 7.1 was used for sample running. Initially, a series of serum dilutions were applied as analytes to find an optimum refractive index. It was observed that the refractive index at 1:500 dilution was within the detection limit among the series of serial dilutions (Fig. 6a). Therefore, 1:500 dilution of different experimental samples was applied as standard for further comparative analysis between Fubc immune response with and without Freund’s adjuvant. Serum control (the serum collected before immunisation) sample with the identical dilution was also applied to find the basal (non-specific) immune response of serum. Finally, refractive index was then analysed from association and dissociation curve of the SPR sensorgram.
Accession number
The Fubc synthetic gene sequence was deposited in GenBank database with accession number MN781186.
Results
Designing of recombinant Fubc antigen
Generally, dengue envelope (E) folded into three distinct domains (designated by domain I, II and III), membrane proximal stem and a transmembrane anchor (Klein et al. 2013). Throughout these structural element, four highly conserved regions have been identified by in silico sequence analysis, and two of them were found in the domain II with a very less informational entropy (Rathore et al. 2019). Further studies have revealed that these conserved regions are the part of previously characterised flavivirus fusion (Fu) and bc loop (Kuhn et al. 2002). During the process of endocytosis, this hydrophobic fusion loop remains buried at the dimer interface in the prefusion state and forms cluster into larger hydrophobic surface at one end to form trimer at later state that finally initiates membrane fusion (Klein et al. 2013). In addition to this structural property, Fu and bc regions consist some of the most potent antigenic epitopes that were identified by DENV neutralising conformation sensitive anti-DENV hMAbs (Goncalvez et al. 2007; Costin et al. 2013; Yamanaka et al. 2013). Therefore, these two conserved Fu and bc loops have been selected to design a recombinant Fubc antigenic protein for the development of dengue subunit vaccine.
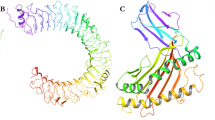
In this study, an alignment of the domain II amino acid sequences of DENV1–4 envelope proteins spanning residues from 61 to 120 was used to find optimum conserved sequence for the development of Fubc fusion protein (Fig. 1a). The structural details of truncated Fubc protein compare to whole envelope in dengue and Fu, and bc peptides were also analysed by modelling each of their three dimensional structure. It reveals that the presence of three anti-parallel β-strands and one disulphide linkage play a crucial role in preserving the three dimensional conformations of truncated Fubc protein same as in original DENV envelope protein (Fig. 1b, c). Ironically, due to the absence of anti-parallel β-strands and a single protein frame of Fu and bc loops, these two separately expressed peptides fail to form similar three-dimensional conformation as in original DENV envelop (Fig. 1c). The electrostatic and solvent accessible areas of Fubc truncated protein are also comparable with original dengue envelope. Hence, it is speculated that the recombinant Fubc protein would be an alternative vaccine target of whole dengue envelope.
Sequence conservation and three dimensional conformation of Fu and bc loops in dengue virus envelope (DENV) protein, Fubc protein and as Fu peptide and bc peptides. a Sequence alignment of DENV envelope proteins of all the four serotypes showing conserved Fu and bc loops. b, c Fu loop is shown in orange and bc loop is shown in yellow. b in DENV envelope, the Fu and bc loops are present at the end of domain II and are linked by the di-sulphide linkage that holds both loops together to form a stable structure that can also work as an epitope. c When the structure of Fubc is compared to original DENV envelope, it is plausible that the presence of three anti-parallel β-strands present in Fubc protein (same as DENV protein) plays a crucial role in maintaining the three dimensional conformation of Fubc protein as it is in DENV protein. Another major factor that plays in conformation of Fu and bc loops is the disulphide linkage between the two highly conserved loop in the Fubc protein that ensures the same conformation of DENV envelope as it is shown in b and c, and d due to the lack of anti-parallel β-strands and same protein frame while these peptides are expressed separately, it is unlike to form di-sulphide linkage and fold in the same conformation as reside in DENV envelope and Fubc proteins
Development of recombinant Fubc gene
For in vitro synthesis of short antigenic protein, a recombinant Fubc gene was constructed by assembly PCR using overlapping oligonucleotides, designed from Fu and bc loop encoding DNA sequences of dengue envelope. Therefore, full length of Fubc gene was amplified by using 5ˊ and 3ˊ end primers flanked by EcoRI and XhoI restriction sites. The Fubc gene was then cloned into a TA cloning vector, and the clones were screened by colony PCR using Fubc gene specific end primers. Two positive clones were then confirmed by restriction digestion using EcoRI and XhoI enzymes (Fig. S1a–d). The digested and purified Fubc insert gene was further sub-cloned into pET28a expression vector, and the positive clones were confirmed by restriction digestion (Fig. S2b) and Sanger sequencing.
Expression and purification of recombinant Fubc protein
Primarily, the recombinant Fubc protein expression was observed only in pellet fraction; there was no such Fubc equivalent protein band in supernatant fraction, while they were separated on SDS PAGE (Fig. S2c). Therefore, Fubc expression level was checked further by lowering growth temperature and IPTG concentration, but no significant change was noticed in supernatant fraction. Chaperone-assisted folding system did not help remarkably to express the Fubc recombinant protein in the soluble form. Therefore, the insoluble pellet fraction of Fubc protein was further utilised in mild solubilisation process (Sarker et al. 2019) to recover soluble Fubc protein. Initially, the recovered soluble Fubc protein fraction was purified by using Ni-NTA agarose beads, and the purified protein band was confirmed by Western blot analysis using anti-His tag monoclonal antibody (Fig. S3). Although, Ni-NTA affinity chromatography has not revealed good purification quality and the yield was also not sufficient. Furthermore, gel filtration chromatography was used to recover good quality large-scale recombinant protein, and single distinct peak greater than 20 mAU was observed at approximately 20.57 ml position for all of injected Fubc protein samples (Fig. 2a). After pooling together, the peak fractions were separated on 15% SDS-PAGE, and single bright band was observed at molecular weight 11.5 kDa (Fig. 2b).
Purification of recombinant Fubc protein by size exclusion chromatography. a FPLC chromatogram of Fubc protein expressed in E. coli inclusion bodies. Three distinct peaks at 19.88 ml, 20.57 ml and 22.57 ml position were collected separately. b Gel image of un-purified and purified Fubc protein sample separated on 15% SDS-PAGE. Lanes 1 and 2 represent the un-purified sample and lanes 3 and 4 represent purified protein sample collected from peak at position 20.57 ml fraction
Structural studies of recombinant Fubc protein
Originally, the Fu and bc loop of dengue envelope is composed of three anti-parallel β-sheets. And the formation of recombinant Fubc secondary structure was characterised here by negative bending at 208 nm and 222 nm wavelength (Kumagai et al. 2017). The CD spectrum of recombinant Fubc protein has showed significantly decreased spectral peaks around 222 and 208 nm that signify the formation of β-sheet (Fig. 3). In addition, it was noticed that the acquired CD spectrum of Fubc at a protein concentration of 0.1 mg/ml in 10 mM PBS buffer was adequately intense (Fig. 2). Therefore, it can be inferred from Fig. 3 (lower panel) that Fubc at concentrations of 0.2 mg/mL and 0.1 mg/mL in 10 mM PBS retained the best globular folded state as compared to other conditions.
CD spectra of recombinant Fubc protein at different buffer concentrations were recorded and analysed with CAPITO tool. The graphical output for its area difference method is also shown. The best matching of reference datasets is 0.2 mg/ml and 0.1 mg/ml Fubc in 10 mM PBS while Fubc 0.2 mg/ml and 0.1 mg/ml in 50 mM PBS have not hit with reference data at all. Lower panel shows the CD values at 200 nm plotted against the CD values at 222 nm to deduce the folding state of Fubc at different buffer concentrations
Immune response to the purified Fubc protein in BALB/c mice
From indirect ELISA, it was observed that the serum IgG levels in both male and female groups treated with Fubc proteins were significantly higher than those treated with only adjuvant and PBS control (Fig. 4a, b). In addition, the response with only Fubc recombinant protein (without adjuvant) in the female group was also observed higher than their male counterpart group (Fig. 4b). All of the ELISA data were also found statistically significant by two-way ANOVA (Table S1). Similarly, by SPR assay, high level immune response was also observed for serum injected with recombinant Fubc protein along with adjuvant, and without any adjuvant, the response was also higher than the control (serum with PBS only) (Fig. 6b).
Serum IgG antibody level to purified Fubc recombinant protein in BALB/c mice. a Immune response of four different groups of male mice. Fubc + FA (blue diamond): Primary immunisation of mice with Fubc protein along with complete Freund’s adjuvant, and booster immunisation with a lower dose of Fubc and incomplete Freund’s adjuvant. Fubc without FA (orange full square): Mice immunised without Freund’s adjuvant. Serum control (black up-pointing triangle): Serum obtained from prebleed of mice (blood harvested from mice before immunisation). FA control (yellow capital letter X): Mice immunised with only Freund’s adjuvant without protein. b IgG level of four different groups of female mice
Discussion
In post-dengue infection, most of the circulating antibodies are non-neutralizing and found to be raised against dengue envelope E protein and the prM protein (Wahala and de Silva 2011). Due to the absence of highly specific neutralising antibodies in secondary infections, cross-reactive non-neutralising antibodies usually enhance the dengue severity (De Alwis et al. 2014). According to the revised dengue case classification (DENCO), 2 to 3% of secondary infections with another serotype causes life threating dengue with warning signs (DWS+) and severe dengue (SD). It was reported that serotype-cross-reactive non-neutralising antibodies enhance the entry of dengue genome into Fc receptor-bearing monocyte cells and promote disease severity by a process known as antibody-depended enhancement (ADE) (Castaño-Osorio et al. 2019; Flipse et al. 2013). That means nature of the antibody response to DENV is most likely to play a major role in defining disease outcome. Therefore, it is predictable that antibodies that recognise specific neutralizing epitopes help in virus clearance and reduce symptoms; however, antibodies that recognise non-neutralising epitopes lead to more severe forms of disease like DWS+/SD. Hence, there is an urgent need for an advanced vaccine which could generate highly specific and cross-neutralising antibody (Costin et al. 2013).
Recently, several attempts have been made towards the development of a potent dengue vaccine. The most advanced candidate, Dengvaxia (CYD-TDV), was licenced in some of the dengue endemic countries (Imai and Ferguson 2018). However, it was revealed risky in children or naïve dengue patient with severe infection as they were vaccinated (Arredondo-García et al. 2018). Therefore, considering safety issues, production of recombinant subunit vaccine with efficient immune protective properties is looking attractive (Govindarajan et al. 2015). Meanwhile, an admixture of four live attenuated recombinant dengue vaccine TV003/TV005 have completed Phase III clinical trial and licenced to several manufacturers including Butantan, VaBiotech and Merk (Whitehead et al. 2017). Moreover, some recombinant tetravalent vaccines (e.g. DEN-80E, TVDV) expressing the prM and E genes of each of the four DENV serotypes from plasmid DNA, have already completed phase I clinical trial (Govindarajan et al. 2015; Danko et al. 2018). It has also been shown that the recombinant dengue envelope domain III can inhibit dengue infectivity, and induce dengue-neutralising immunoglobulin in mice (Hermida et al. 2006). In addition, a number of antibodies which were raised in mice and chimpanzees against dengue domain II fusion loop were found cross-reactive to other flaviviruses (Goncalvez et al. 2004; Stiasny et al. 2006). Although, the hMABs specific to dengue domain II fusion loop were not found equally effective on other flaviviruses including WNV, YFV and other DENV strain (Costin et al. 2013). Therefore, it was conjectured that adjacent to the fusion loop, additional contact residues of original domain II surface structure might be involved to raise cross-neutralizing antibodies.
Several studies have identified that adjacent to the fusion loop, another similar loop exists in most of the flaviviral (e.g. WNV, TBE, JEV, YFV) envelope (Fibriansah and Lok, 2016; Li et al. 2019). Our previous bioinformatics studies have also shown that the envelope of all DENV serotype consists of four conserved regions (> 90%), and two of them were found in domain II, in which one is fusion loop (Fu) with more than 99% conservation and another is its nearby bc loop (Fig. 1a) (Rathore et al. 2019). Therefore, these two highly conserved loops were targeted in this study for the development of a fusion protein. By using reverse DNA technology, we have previously shown the structural integrity (Fig. 1b–d) and binding specificity of Fubc protein with an anti-fusion loop scFv antibody, derived from dengue and WNV-specific MAb E53 (Rodenhuis-Zybert et al. 2011; Rathore et al. 2019).
The very early challenge of subunit vaccine design is to produce large quantities of functional protein. Hereby, we have successfully optimised the expression and purification methods for the production of large-scale high-quality recombinant Fubc protein. The E. coli expression system was used here, and the recombinant protein was found to be expressed in higher quantity, though most of the protein was extracted initially in pellet fraction. No significant change was noticed by altering regular growth temperature and inducer concentration. Therefore, we have utilised the mild-solubilisation methodology to recover soluble Fubc protein from insoluble pellet protein. For purification, firstly, we have used convenient Ni-NTA affinity purification method, and by adjusting the lysis and elution buffer, we were able to purify recombinant Fubc protein to some extent but the quality and quantity was not sufficient. Finally, to scale up the quality protein production, size exclusion chromatography was used in AKTA FPLC system. To confirm the recombinant protein expression, simple western blot was performed by using anti-His monoclonal antibody as primary. Furthermore, in vitro structural integrity and functional authenticity of the experimental Fubc protein were also verified by CD spectroscopy (Fig. 3) and indirect ELISA (Fig. 4).
However, most of the hMABs identified earlier from dengue patient serum were predominantly cross-reactive and recognise epitopes containing highly conserved residues at the Fu loop of domain II (Lai et al. 2008). However, having such sequence homology in Fu loop of all flaviviruses, these hMABs are non-neutralizing against heterologous serotypes (Lai et al. 2008; Smith et al. 2013) and thus was found to be responsible for ADE in animals (Goncalvez et al. 2007). Previously, it has also been stated that this scenario might happen due to the cryptic nature and poor accessibility of the fusion loop epitope on the surface of the mature virion (Lai et al. 2008; Cherrier et al. 2009). However, in addition to the partially exposed fusion loop on immature flaviviruses, some neutralizing hMAbs were also found to bind with bc loop (Costin et al. 2013). Therefore, it is suggestive that along with conserved fusion loop, adjacent less conserved linker sequence and bc loop might be required to be exposed as a whole to generate effective cross-neutralizing immunity. Our current mouse immunisation experiment also supports this idea as it was observed that the antibody response to full length Fubc protein was stronger than the response elicited by the Fu and Bc peptides in separate (Figs. 4 and 5).
Comparative analysis of immune response between Fubc recombinant protein and Fu, bc peptides: Serum IgG antibody level was recorded by ELISA using five different groups of serum samples. (1) Fubc protein + FA (blue diamond), (2) Fubc protein without FA (orange full square), (3) Fu, bc peptides + FA (black up-pointing triangle) (4) Fu, bc peptide without FA (yellow capital letter X), and (5) serum control as a baseline (blue asterisk)
Moreover, without Freund’s adjuvant, recombinant Fubc protein was found immunogenic, and the response in female mice was stronger than in male (Figs. 4 and 5). These observations were also symmetrical with other studies where it was stated that female mice have a better immune response due to the higher number of leukocytes occupying the naive peritoneal and pleural cavities, and also have a number of T- and B-lymphocytes as well as macrophages (Terres et al. 1968; Weinstein et al. 1984; Klein et al. 2015). Therefore, we have used female mice serum samples for further qualitative ELISA and SPR test to quantify immune response of recombinant Fubc protein. It was noticed that IgG antibody response to Fu, and bc peptides were significantly lower than the response observed to Fubc recombinant protein, and the immune response achieved to Fu and Bc peptide with Freund’s adjuvant was significantly higher as compared to the peptides without adjuvant and serum control (Fig. 5). Two-way ANOVA was also performed to analyse the significance of IgG immune responses between Fubc + FA and Fubc without FA; Fu, bc Peptide + FA and Fu, bc Peptide without FA. From F values and P values obtained by ANOVA test, it can be interpreted that the difference between Fubc protein with Freund’s adjuvant and Fu, bc Peptide with Freund’s adjuvant were significantly higher than protein and peptides injected without Freund’s adjuvant (Table S2). Also, the IgG response to recombinant Fubc protein was recorded better than Fu and bc peptides in both cases either with or without adjuvant. In addition, the p value of IgG immune response between Fubc protein with FA and without FA was found insignificant (p = 0.1740 > 0.05) that suggests that Fubc without any adjuvant is sufficient to elicit significant immune response (Table S2). Consistently, it was observed by SPR experiment that the refractive index of Fubc + FA was significantly higher than Fubc + without FA and serum control (Fig. 6b).
Since, the newly developed Fubc recombinant protein expresses vastly in E. coli and induces significant immune response in mice, it might be a good agent of dengue subunit vaccine development. Due to the presence of highly conserved fusion loop epitope, overlapping less conserved linker sequences and also bc epitope, this fusion protein might induce cross-neutralisation immunity against heterologous dengue serotypes. Though, still a lot of investigations, like the evaluation of a proper adjuvant to induce robust immune response, the memory immune response generated against the Fubc protein both by humoral and cell-mediated immunity, and whether this memory response will provide protection against a secondary encounter with DENV, are required before going clinical trial. Nevertheless, the production process and immune response of this fusion protein would provide new insight for the development of dengue subunit vaccine.
References
Arredondo-García JL, Hadinegoro SR, Reynales H, Chua MN, Rivera Medina DM, Chotpitayasunondh T, Zambrano B (2018) Four-year safety follow-up of the tetravalent dengue vaccine efficacy randomized controlled trials in Asia and Latin America. Clin Microbiol Infect 24:755–763. https://doi.org/10.1016/j.cmi.2018.01.018
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496:504–507. https://doi.org/10.1038/nature12060
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI (2012) Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6:e1760. https://doi.org/10.1371/journal.pntd.0001760
Castaño-Osorio JC, Giraldo-Garcia AM, Giraldo MI. (2019) Current status of vaccines against dengue virus. Dengue fever - a resilient threat in the face of innovation IntechOpen Book chapter 9. https://doi.org/10.5772/intechopen.80820
Cherrier MV, Kaufmann B, Nybakken GE, Lok S-M, Warren JT, Chen BR, Nelson CA, Kostyuchenko VA, Holdaway HA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG, Fremont DH (2009) Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. EMBO J 28:3269–3276. https://doi.org/10.1038/emboj.2009.245
Coller B-AG, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH (2011) The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 29:7267–7275. https://doi.org/10.1016/j.vaccine.2011.07.021
Costin JM, Zaitseva E, Kahle KM, Nicholson CO, Rowe DK, Graham AS, Bazzone LE, Hogancamp G, Figueroa Sierra M, Fong RH, Yang S-T, Lin L, Robinson JE, Doranz BJ, Chernomordik LV, Michael SF, Schieffelin JS, Isern S (2013) Mechanistic study of broadly neutralizing human monoclonal antibodies against dengue virus that target the fusion loop. J Virol 87:52–66. https://doi.org/10.1128/JVI.02273-12
Danko JR, Beckett CG, Porter KR (2011) Development of dengue DNA vaccines. Vaccine 29:7261–7266. https://doi.org/10.1016/j.vaccine.2011.07.019
Danko JR, Kochel T, Teneza-Mora N, Luke TC, Raviprakash K, Sun P, Porter KR (2018) Safety and immunogenicity of a tetravalent dengue DNA vaccine administered with a cationic lipid-based adjuvant in a phase 1 clinical trial. Am J Trop Med Hyg 98:849–856. https://doi.org/10.4269/ajtmh.17-0416
De Alwis R, Williams KL, Schmid MA, Lai C-Y, Patel B, Smith SA, Crowe JE, Wang W-K, Harris E, de Silva AM (2014) Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog 10:e1004386. https://doi.org/10.1371/journal.ppat.1004386
Diaz C, Lin L, Martinez LJ, Eckels KH, Campos M, Jarman RG, De La Barrera R, Lepine E, Toussaint JF, Febo I, Innis BL, Thomas SJ, Schmidt AC (2018) Phase I randomized study of a tetravalent dengue purified inactivated vaccine in healthy adults from Puerto Rico. Am J Trop Med Hyg 98:1435–1443. https://doi.org/10.4269/ajtmh.17-0627
Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS (2011) Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 29:7242–7250. https://doi.org/10.1016/j.vaccine.2011.07.023
Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, de Silva AM, Harris E, Crowe JE, Lok SM (2015) Dengue virus cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 349:88–91. https://doi.org/10.1126/science.aaa8651
Fibriansah G, Lok SM (2016) The development of therapeutic antibodies against dengue virus. Antivir Res 128:7–19. https://doi.org/10.1016/j.antiviral.2016.01.002
Flipse J, Wilschut J, Smit JM (2013) Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic 14:25–35. https://doi.org/10.1111/tra.12012
Gallichotte EN, Widman DG, Yount BL, Wahala WM, Durbin A, Whitehead S, Baric, RS 2015 A new quaternary structure epitope on dengue virus serotype 2 is the target of durable type-specific neutralizing antibodies. mBio 6. https://doi.org/10.1128/mbio.01461-15
Garçon N, Leroux-Roels G, Cheng W-F (2011) Vaccine adjuvants. Persp Vaccinol 1:89–113. https://doi.org/10.1016/j.pervac.2011.05.004
Giraldo-García AM, Castaño-Osorio JC (2019) Development of a dengue vaccine and its use in pregnant women. Curr Trop Med Rep. https://doi.org/10.1007/s40475-019-00192
Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai C-J (2007) Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A 104:9422–9427. https://doi.org/10.1073/pnas.0703498104
Goncalvez AP, Purcell RH, Lai C-J (2004) Epitope determinants of a chimpanzee fab antibody that efficiently cross-neutralizes dengue type 1 and type 2 viruses map to inside and in close proximity to fusion loop of the dengue type 2 virus envelope glycoprotein. J Virol 78:12919–12928. https://doi.org/10.1128/JVI.78.23.12919-12928.2004
Govindarajan D, Meschino S, Guan L, Clements DE, ter Meulen JH, Casimiro DR, Coller B-AG, Bett AJ (2015) Preclinical development of a dengue tetravalent recombinant subunit vaccine: immunogenicity and protective efficacy in nonhuman primates. Vaccine 33:4105–4116. https://doi.org/10.1016/j.vaccine.2015.06.067
Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J (2011) From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 29:7229–7241. https://doi.org/10.1016/j.vaccine.2011.06.094
Guy B, Briand O, Lang J, Saville M, Jackson N (2015) Development of the Sanofi Pasteur tetravalent dengue vaccine: one more step forward. Vaccine 33:7100–7111. https://doi.org/10.1016/j.vaccine.2015.09.108
Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB (2016) Dengue infection. Nat Rev Dis Primers 2:16055. https://doi.org/10.1038/nrdp.2016.55
Guzman MG, Hermida L, Bernardo L, Ramirez R, Guillén G (2010) Domain III of the envelope protein as a dengue vaccine target. Expert Rev Vaccines 9:137–147. https://doi.org/10.1586/erv.09.139
Hermida L, Bernardo L, Martín J, Alvarez M, Prado I, López C, de la C Sierra B, Martínez R, Rodríguez R, Zulueta A, Pérez AB, Lazo L, Rosario D, Guillén G, Guzmán MG (2006) A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine 24:3165–3171. https://doi.org/10.1016/j.vaccine.2006.01.036
Higgins D, Marshall JD, Traquina P, Van Nest G, Livingston BD (2007) Immunostimulatory DNA as a vaccine adjuvant. Expert Rev Vaccines 6:747–759. https://doi.org/10.1586/14760584.6.5.747
Imai N, Ferguson NM (2018) Targeting vaccinations for the licensed dengue vaccine: considerations for serosurvey design. PLoS One:13. https://doi.org/10.1371/journal.pone.0199450
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim Biophys Acta 1751:119–139. https://doi.org/10.1016/j.bbapap.2005.06.005
Klein DE, Choi JL, Harrison SC (2013) Structure of a dengue virus envelope protein late-stage fusion intermediate. J Virol 87(4):2287–2293. https://doi.org/10.1128/JVI.02957-12
Klein SL, Marriott I, Fish EN (2015) Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 109:9–15. https://doi.org/10.1093/trstmh/tru167
Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH (2002) Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725
Kumagai PS, Araujo APU, Lopes JLS (2017) Going deep into protein secondary structure with synchrotron radiation circular dichroism spectroscopy. Biophys Rev 9:517–527. https://doi.org/10.1007/s12551-017-0314-2
Lai C-Y, Tsai W-Y, Lin S-R, Kao C-L, Hu H-P, King C-C, Wu H-C, Chang G-J, Wang W-K (2008) Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol 82:6631–6643. https://doi.org/10.1128/JVI.00316-08
Li L, Meng W, Horton M, DiStefano DR, Thoryk EA, Pfaff JM, Wang Q, Salazar GT, Barnes T, Doranz BJ, Bett AJ, Casimiro DR, Vora KA, An Z, Zhang N (2019) Potent neutralizing antibodies elicited by dengue vaccine in rhesus macaque target diverse epitopes. PLoS Pathog 15:e1007716. https://doi.org/10.1371/journal.ppat.1007716
Liu G, Song L, Beasley DWC, Putnak R, Parent J, Misczak J, Li H, Reiserova L, Liu X, Tian H, Liu W, Labonte D, Duan L, Kim Y, Travalent L, Wigington D, Weaver B, Tussey L (2015) Immunogenicity and efficacy of flagellin-envelope fusion dengue vaccines in mice and monkeys. Clin Vaccine Immunol 22:516–525. https://doi.org/10.1128/CVI.00770-14
Maira-Litrán T (2017) Immunization of mice. Curr Protoc Mol Biol 117:11.4.1–11.4.11. https://doi.org/10.1002/cpmb.30
Martina BEE, Koraka P, Osterhaus ADME (2009) Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 22:564–581. https://doi.org/10.1128/CMR.00035-09
Miles AJ, Wallace BA (2006) Synchrotron radiation circular dichroism spectroscopy of proteins and applications in structural and functional genomics. Chem Soc Rev 35:39–51. https://doi.org/10.1039/b316168b
Modis Y, Ogata S, Clements D, Harrison SC (2004) Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313–319. https://doi.org/10.1038/nature02165
Murphy BR, Whitehead SS (2011) Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 29:587–619. https://doi.org/10.1146/annurev-immunol-031210-101315
Nayak V, Dessau M, Kucera K, Anthony K, Ledizet M, Modis Y (2009) Crystal structure of dengue virus type 1 envelope protein in the postfusion conformation and its implications for membrane fusion. J Virol 83:4338–4344. https://doi.org/10.1128/JVI.02574-08
Pang T, Gubler D, Goh DYT, Ismail Z (2018) Dengue vaccination: a more balanced approach is needed. Lancet 391:654. https://doi.org/10.1016/S0140-6736(18)30245-9
Rathore AS, Sarker A, Gupta RD (2019) Designing antibody against highly conserved region of dengue envelope protein by in silico screening of scFv mutant library. PLoS One 14:e0209576. https://doi.org/10.1371/journal.pone.0209576
Rodenhuis-Zybert IA, Moesker B, da Silva Voorham JM, van der Ende-Metselaar H, Diamond MS, Wilschut J, Smit JM (2011) A fusion-loop antibody enhances the infectious properties of immature flavivirus particles. J Virol 85:11800–11808. https://doi.org/10.1128/JVI.05237-11
Sarker A, Rathore AS, Gupta RD (2019) Evaluation of scFv protein recovery from E. coli by in vitro refolding and mild solubilization process. Microb Cell Factories 18:5. https://doi.org/10.1186/s12934-019-1053-9
Smith SA, de Alwis AR, Kose N, Harris E,1 Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, Austin SK, Sukupolvi-Petty S, Diamond MS, Crowe JE (2013) The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain ii of the envelope protein. mBio 4. https://doi.org/10.1128/mBio.00873-13
Stiasny K, Kiermayr S, Holzmann H, Heinz FX (2006) Cryptic properties of a cluster of dominant flavivirus cross-reactive antigenic sites. J Virol 80:9557–9568. https://doi.org/10.1128/JVI.00080-06
Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, Fremont DH, Diamond MS (2010) Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol 84:9227–9239. https://doi.org/10.1128/JVI.01087-10
Terres G, Morrison SL, Habicht GS (1968) A quantitative difference in the immune response between male and female mice. Exp Biol Med 127:664–667. https://doi.org/10.3181/00379727-127-32768
Wahala WMPB, de Silva AM (2011) The human antibody response to dengue virus infection. Viruses 3:2374–2395. https://doi.org/10.3390/v3122374
Weinstein Y, Ran S, Segal S (1984) Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol 132:656–661
Whitehead SS, Durbin AP, Pierce KK, Elwood D, McElvany BD, Fraser EA, Carmolli MP (2017) In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic insubjects with flavivirus exposure prior to vaccination. PLoS Negl Trop Dis 11:e0005584. https://doi.org/10.1371/journal.pntd.0005584
Yamanaka A, Kotaki T, Konishi E (2013) A mouse monoclonal antibody against dengue virus type 1 Mochizuki strain targeting envelope protein domain II and displaying strongly neutralizing but not enhancing activity. J Virol 87:12828–12837. https://doi.org/10.1128/JVI.01874-13
Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG (2004) Conformational changes of the flavivirus e glycoprotein. Structure 12:1607–1618. https://doi.org/10.1016/j.str.2004.06.019
Acknowledgements
ASR thanks University Grant Commission, India for providing Senior Research Fellowship. AS thanks South Asian University for Ph.D. Fellowship.
Availability of data and material
All the relevant data used to support the findings of this study are included within the article.
Funding
This study was funded by the Defence Research and Development Organization (Project No. LSRB-297) and Science and Engineering Research Board (Project No. EMR/2016/007246).
Author information
Authors and Affiliations
Contributions
R.D.G. conceived the study. R.D.G., A.S.R. and A.S. designed the experiments. A.S.R and A.S. performed the experiments. R.D.G., A.S.R. and A.S. analysed the data and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All the required ethical approvals have been taken before performing the experiments.
Competing interests
The authors declare that they have no competing interests.
Declaration
All the authors have given consent for the publication of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1654 kb)
Rights and permissions
About this article
Cite this article
Rathore, A.S., Sarker, A. & Gupta, R.D. Production and immunogenicity of Fubc subunit protein redesigned from DENV envelope protein. Appl Microbiol Biotechnol 104, 4333–4344 (2020). https://doi.org/10.1007/s00253-020-10541-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10541-y