Abstract
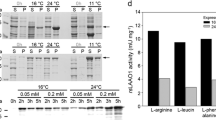
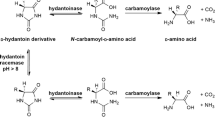
l-Amino acid oxidases (LAAOs) are flavoproteins, which use oxygen to deaminate l-amino acids and produce the corresponding α-keto acids, ammonia, and hydrogen peroxide. Here we describe the heterologous expression of LAAO4 from the fungus Hebeloma cylindrosporum without signal sequence as fusion protein with a 6His tag in Escherichia coli and its purification. 6His-hcLAAO4 could be activated by exposure to acidic pH, the detergent sodium dodecyl sulfate, or freezing. The enzyme converted 14 proteinogenic l-amino acids with l-glutamine, l-leucine, l-methionine, l-phenylalanine, l-tyrosine, and l-lysine being the best substrates. Methyl esters of these l-amino acids were also accepted. Even ethyl esters were converted but with lower activity. Km values were below 1 mM and vmax values between 19 and 39 U mg−1 for the best substrates with the acid-activated enzyme. The information for an N-terminal aldehyde tag was added to the coding sequence. Co-expressed formylglycine-generating enzyme was used to convert a cysteine residue in the aldehyde tag to a Cα-formylglycine residue. The aldehyde tag did not change the properties of the enzyme. Purified Ald-6His-hcLAAO4 was covalently bound to a hexylamine resin via the Cα-formylglycine residue. The immobilized enzyme could be reused repeatedly to generate phenylpyruvate from l-phenylalanine with a total turnover number of 17,600 and was stable for over 40 days at 25 °C.






Similar content being viewed by others
References
Belval L, Marquette A, Mestre P, Piron MC, Demangeat G, Merdinoglu D, Chich JF (2015) A fast and simple method to eliminate Cpn60 from functional recombinant proteins produced by E-coli Arctic Express. Protein Expr Purif 109:29–34. https://doi.org/10.1016/j.pep.2015.01.009
Benjdia A, Deho G, Rabot S, Berteau O (2007) First evidences for a third sulfatase maturation system in prokaryotes from E. coli aslB and ydeM deletion mutants. Febs L 581:1009–1014. https://doi.org/10.1016/j.febslet.2007.01.076
Bifulco D, Pollegioni L, Tessaro D, Servi S, Molla G (2013) A thermostable L-aspartate oxidase: a new tool for biotechnological applications. Appl Microbiol Biotechnol 97:7285–7295. https://doi.org/10.1007/s00253-013-4688-1
Blaser HU, Studer M (1999) Critical issues for using enantioselective catalysis for fine chemicals production. Chirality 11:459–464. https://doi.org/10.1002/(sici)1520-636x(1999)11:5/6<459::aid-chir17>3.0.co;2-n
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Camarero JA (2008) Recent developments in the site-specific immobilization of proteins onto solid supports. Biopolymers 90:450–458. https://doi.org/10.1002/bip.20803
Candiano G, Bruschi M, Musante L, Santucci L, Ghiggeri GM, Carnemolla B, Orecchia P, Zardi L, Righetti PG (2004) Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 25:1327–1333. https://doi.org/10.1002/elps.200305844
Carrico IS, Carlson BL, Bertozzi CR (2007) Introducing genetically encoded aldehydes into proteins. Nat Chem Biol 3(6):321–322. https://doi.org/10.1038/nchembio878
Cheng CH, Yang CA, Liu SY, Lo CT, Huang HC, Liao FC, Peng KC (2011) Cloning of a novel L-amino acid oxidase from Trichoderma harzianum ETS 323 and bioactivity analysis of overexpressed L-amino acid oxidase. J Agric Food Chem 59:9142–9149. https://doi.org/10.1021/jf201598z
Coles CJ, Edmondson DE, Singer TP (1977) Reversible inactivation of L-amino-acid oxidase - properties of 3 conformational forms. J Biol Chem 252:8035–8039
Curti B, Massey V, Zmudka M (1968) Inactivation of snake venom L-amino acid oxidase by freezing. J Biol Chem 243:2306–2314
D'Arrigo P, Allegretti C, Fiorati A, Piubelli L, Rosini E, Tessaro D, Valentino M, Pollegioni L (2015) Immobilization of L-aspartate oxidase from Sulfolobus tokodaii as a biocatalyst for resolution of aspartate solutions. Catal Sci Technol 5:1106–1114. https://doi.org/10.1039/c4cy00968a
Davis MA, Askin MC, Hynes MJ (2005) Amino acid catabolism by an areA-regulated gene encoding an L-amino acid oxidase with broad substrate specificity in Aspergillus nidulans. Appl Environ Microbiol 71:3551–3555. https://doi.org/10.1128/aem.71.7.3551-3555.2005
Du XY, Clemetson KJ (2002) Snake venom L-amino acid oxidases. Toxicon 40:659–665. https://doi.org/10.1016/s0041-0101(02)00102-2
Espin JC, Wichers HJ (1999) Activation of a latent mushroom (Agaricus bisporus) tyrosinase isoform by sodium dodecyl sulfate (SDS). kinetic properties of the SDS-activated isoform. J Agric Food Chem 47:3518–3525. https://doi.org/10.1021/jf981275p
Ferrer M, Chernikova TN, Timmis KN, Golyshin PN (2004) Expression of a temperature-sensitive esterase in a novel chaperone-based Escherichia coli strain. Appl Environ Microbiol 70:4499–4504. https://doi.org/10.1128/aem.70.8.4499-4504.2004
Glick BR (1995) Metabolic load and heterologous gene-expression. Biotechnol Adv 13:247–261. https://doi.org/10.1016/0734-9750(95)00004-a
Hahn K, Hertle Y, Bloess S, Kottke T, Hellweg T, von Mollard G (2017a) Activation of recombinantly expressed L-amino acid oxidase from Rhizoctonia solani by sodium dodecyl sulfate. Molecules 22(12). https://doi.org/10.3390/molecules22122272
Hahn K, Neumeister K, Mix A, Kottke T, Gröger H, von Mollard G (2017b) Recombinant expression and characterization of a L-amino acid oxidase from the fungus Rhizoctonia solani. Appl Microbiol Biotechnol 101:2853–2864. https://doi.org/10.1007/s00253-016-8054-y
Hossain GS, Li J, Shin H-d DG, Liu L, Chen J (2014) L-Amino acid oxidases from microbial sources: types, properties, functions, and applications. Appl Microbiol Biotechnol 98:1507–1515. https://doi.org/10.1007/s00253-013-5444-2
Jian H, Wang YW, Bai Y, Li R, Gao RJ (2016) Site-specific, covalent immobilization of dehalogenase ST2570 catalyzed by formylglycine-generating enzymes and its application in batch and semi-continuous flow reactors. Molecules 21. https://doi.org/10.3390/molecules21070895
Kanade SR, Paul B, Rao AGA, Gowda LR (2006) The conformational state of polyphenol oxidase from field bean (Dolichlos lablah) upon SDS and acid-pH activation. Biochem J 395:551–562. https://doi.org/10.1042/bj20051509
Kasai K, Ishikawa T, Komata T, Fukuchi K, Chiba M, Nozaka H, Nakamura T, Sato T, Miura T (2010) Novel L-amino acid oxidase with antibacterial activity against methicillin-resistant Staphylococcus aureus isolated from epidermal mucus of the flounder Platichthys stellatus. FEBS J 277:453–465. https://doi.org/10.1111/j.1742-4658.2009.07497.x
Kearney EB, Singer TP (1951) The L-amino acid oxidases of snake venom. III. Reversible inactivation of l-amino acid oxidases. Arch Biochem Biophys 33:377–396. https://doi.org/10.1016/0003-9861(51)90125-7
Kenten RH (1957) Latent phenolase in extracts of broad-bean (Vicia faba L.) leaves. Activation by acid and alkali. Biochem J 67:300–307. https://doi.org/10.1042/bj0670300
Kenten RH (1958) Latent phenolase in extracts of broad-bean (Vicia faba L.) leaves. 2. Activation by anionic wetting agents. Biochem J 68:244–251. https://doi.org/10.1042/bj0680244
Krüger T, Weiland S, Falck G, Gerlach M, Boschanski M, Alam S, Muller KM, Dierks T, Sewald N (2018) Two-fold bioorthogonal derivatization by different formylglycine-generating enzymes. Angew Chem Int Ed 57:7245–7249. https://doi.org/10.1002/anie.201803183
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liese A, Hilterhaus L (2013) Evaluation of immobilized enzymes for industrial applications. Chem Soc Rev 42:6236–6249. https://doi.org/10.1039/c3cs35511j
Liu L, Hossain GS, Shin HD, Li JH, Du GC, Chen J (2013) One-step production of alpha-ketoglutaric acid from glutamic acid with an engineered L-amino acid deaminase from Proteus mirabilis. J Biotechnol 164:97–104. https://doi.org/10.1016/j.jbiotec.2013.01.005
Masuko M, Iwasaki H (1984) Activation of nitrite reductase in a cell-free system from the denitrifier Alcaligenes sp by freeze-thawing. Plant Cell Physiol 25:439–446
Masuko M, Iwasaki H, Sakurai T, Suzuki S, Nakahara A (1985) Effects of freezing on purified nitrite reductase from a denitrifier, Alcaligenes sp ncib 11015. J Biochem 98:1285–1291. https://doi.org/10.1093/oxfordjournals.jbchem.a135395
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microbial Technol 40:1451–1463. https://doi.org/10.1016/j.enzmictec.2007.01.018
McIlvaine TC (1921) A buffer solution for colorimetric comparison. J Biol Chem 49:183–186
Moore BM, Flurkey WH (1990) Sodium dodecyl-sulfate activation of a plant polyphenoloxidase—effect of sodium dodecyl-sulfate on enzymatic and physical characteristics of purified broad bean polyphenoloxidase. J Biol Chem 265:4982–4988
Nakai K, Horton P (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24:34–35. https://doi.org/10.1016/s0968-0004(98)01336-x
Nuutinen JT, Timonen S (2008) Identification of nitrogen mineralization enzymes, L-amino acid oxidases, from the ectomycorrhizal fungi Hebeloma spp. and Laccaria bicolor. Mycol Res 112:1453–1464. https://doi.org/10.1016/j.mycres.2008.06.023
Nuutinen JT, Marttinen E, Soliymani R, Hilden K, Timonen S (2012) L-Amino acid oxidase of the fungus Hebeloma cylindrosporum displays substrate preference towards glutamate. Microbiology 158:272–283. https://doi.org/10.1099/mic.0.054486-0
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth 8:785–786. https://doi.org/10.1038/nmeth.1701
Pollegioni L, Molla G (2011) New biotech applications from evolved D-amino acid oxidases. Trends Biotechnol 29:276–283. https://doi.org/10.1016/j.tibtech.2011.01.010
Pollegioni L, Motta P, Molla G (2013) L-Amino acid oxidase as biocatalyst: a dream too far ? Appl Microbiol Biotechnol 97:9323–9341. https://doi.org/10.1007/s00253-013-5230-1
Rios GM, Belleville MP, Paolucci D, Sanchez J (2004) Progress in enzymatic membrane reactors—a review. J Membr Sci 242:189–196. https://doi.org/10.1016/j.memsci.2003.06.004
Seelbach K, vanDeurzen MPJ, vanRantwijk F, Sheldon RA, Kragl U (1997) Improvement of the total turnover number and space-time yield for chloroperoxidase catalyzed oxidation. Biotechnol Bioeng 55:283–288
Sörensen SPL (1909) Enzymstudien. II. Mitteilung. Über die Messung und die Bedeutung der Wasserstoffion Konzentration bei enzymatischen Prozessen. Biochem Z 21:131
Sungkeeree P, Whangsuk W, Sallabhan R, Dubbs J, Mongkolsuk S, Loprasert S (2017) Efficient removal of toxic phthalate by immobilized serine-type aldehyde-tagged esterase G. Process Biochem 63:60–65. https://doi.org/10.1016/j.procbio.2017.09.009
Tong HC, Chen W, Shi WY, Qi FX, Dong XZ (2008) SO-LAAO, a novel L-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J Bacteriol 190:4716–4721. https://doi.org/10.1128/jb.00363-08
Wang AM, Du FC, Wang F, Shen YQ, Gao WF, Zhang PF (2013) Convenient one-step purification and immobilization of lipase using a genetically encoded aldehyde tag. Biochem Eng J 73:86–92. https://doi.org/10.1016/j.bej.2013.02.003
Wittenberg C, Triplett EL (1985) A detergent-activated tyrosinase from Xenopus laevis. 2. Detergent activation and binding. J Biol Chem 260:2542–2546
Yang HC, Johnson PM, Ko KC, Kamio M, Germann MW, Derby CD, Tai PC (2005) Cloning, characterization and expression of escapin, a broadly antimicrobial FAD-containing L-amino acid oxidase from ink of the sea hare Aplysia californica. J Exp Biol 208:3609–3622. https://doi.org/10.1242/jeb.01795
Yang CA, Cheng CH, Lo CT, Liu SY, Lee JW, Peng KC (2011) A novel L-amino acid oxidase from Trichoderma harzianum ETS 323 associated with antagonism of Rhizoctonia solani. J Agric Food Chem 59:4519–4526. https://doi.org/10.1021/jf104603w
Zhao XS, Bao XY, Guo WP, Lee FY (2006) Immobilizing catalysts on porous materials. Mater Today 9:32–39. https://doi.org/10.1016/s1369-7021(06)71388-8
Acknowledgements
We thank Marco Wißbrock and Annalena Lausch for excellent technical assistance. The pET14b-mtbFGE-His6 plasmid was kindly provided by David Rabuka (Redwood Bioscience, Emeryville, USA).
Funding
This study was funded by Universität Bielefeld as a strategic project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bloess, S., Beuel, T., Krüger, T. et al. Expression, characterization, and site-specific covalent immobilization of an L-amino acid oxidase from the fungus Hebeloma cylindrosporum. Appl Microbiol Biotechnol 103, 2229–2241 (2019). https://doi.org/10.1007/s00253-018-09609-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-09609-7