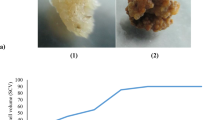
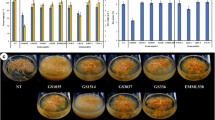
Abstract
Cobalt nitrate, nickel sulphate, hydrogen peroxide, sodium nitroprusside, and culture filtrates of Pseudomonas monteili, Bacillus circularans, Trichoderma atroviridae, and Trichoderma harzianum were tested to elicit ginsenoside production in a cell suspension line of Panax quinquefolius. Abiotic elicitors preferentially increased panaxadiols whereas biotic elicitors upregulated the panaxatriol synthesis. Cobalt nitrate (50 μM) increased total ginsenosides content by twofold (54.3 mg/L) within 5 days. It also induced the Rc synthesis that was absent in the control cultures. Elicitation with P. monteili (2.5 % v/v, 5 days) also supported 2.4-fold enhancement in saponin yield. Elicitation by T. atroviridae or hydrogen peroxide induced the synthesis of Rg3 and Rh2 that are absent in ginseng roots. The highest ginsenosides productivity (3.2-fold of control) was noticed in cells exposed to 1.25 % v/v dose of T. atroviridae for 5 days. Treating cells with T. harzianum for 15 days afforded maximum synthesis and leaching (8.1 mg/L) of ginsenoside Rh1.

Similar content being viewed by others
References
Akula R, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Algar E, Gutierrez-Mañero FJ, Bonilla A, Lucas JA, Radzki W, Ramos-Solano B (2012) Pseudomonas fluorescens N21. metabolites enhance secondary metabolism isoflavones in soybean (Glycine max) calli cultures. J Agric Food Chem 60:11080–11087
Awad V, Kuvalekar A, Harsulkar A (2014) Microbial elicitation in root cultures of Taverniera cuneifolia (Roth) Arn. for elevated glycyrrhizic acid production. Ind Crop Prod 54:13–16
Biswas T, Ajayakumar PV, Mathur AK, Mathur A (2015) Solvent-based extraction optimisation for efficient ultrasonication-assisted ginsenoside recovery from Panax quinquefolius and P. sikkimensis cell suspension lines. Nat Prod Res 29:1256–1263
Cai Z, Kastell A, Knorr D, Smetanska I (2012) Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep 31:461–477
Chandra S, Chandra R (2011) Engineering secondary metabolite production in hairy roots. Phytochemistry Rev 10:371–395
Chodisetti B, Rao K, Gandi S, Giri A (2013) Improved gymnemic acid production in the suspension cultures of Gymnema sylvestre through biotic elicitation. Plant Biotechnol Rep 7:519–525
Hu X, Neill S, Cai W, Tang Z (2003a) Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid‐induced saponin accumulation in suspension‐cultured cells of Panax ginseng. Physiol Plant 118:414–421
Hu X, Neill SJ, Cai W, Tang Z (2003b) Nitric oxide mediates elicitor-induced saponin synthesis in cell cultures of Panax ginseng. Funct Plant Biol 30:901–907
Huang C, Zhong JJ (2013) Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate. Process Biochem 48:1227–1234
Huang C, Qian ZG, Zhong JJ (2013) Enhancement of ginsenoside biosynthesis in cell cultures of Panax ginseng by N, N′-dicyclohexylcarbodiimide elicitation. J Biotechnol 165:30–36
Jeong GT, Park DH (2006) Enhanced secondary metabolite biosynthesis by elicitation in transformed plant root system. In: Mc Milan JD, Adney WS, Mielenz JR, Klasson KT (eds) Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals. Humana Press, New York, pp 436–446
Karwasara VS, Dixit VK (2013) Culture medium optimization for camptothecin production in cell suspension cultures of Nothapodytes nimmoniana (J. Grah.) Mabberley. Plant Biotechnol Rep 7:357–369
Kim C, Im H, Kim H, Huh H (2001) Accumulation of 2, 5-dimethoxy-1, 4-benzoquinone in suspension cultures of Panax ginseng by a fungal elicitor preparation and a yeast elicitor preparation. Appl Microbiol Biotechnol 56:239–242
Mañero F, Gutiérrez J, Algar E, Martín Gómez MS, Saco Sierra MD, Solano BR (2012) Elicitation of secondary metabolism in Hypericum perforatum by rhizosphere bacteria and derived elicitors in seedlings and shoot cultures. Pharm Biol 50:1201–1209
Mathur A, Shukla YN, Pal M, Ahuj PS, Uniyal GC (1994) Saponin production in callus and cell suspension cultures of Panax quinquefolium. Phytochemistry 35:221–1225
Mathur A, Mathur AK, Uniyal GC, Pal M, Sangwan RS (2001) A process for the development of a stable high yielding callus line of Panax quinquefolium. US Patent 6326202 B1
Mathur A, Gangwar A, Mathur AK, Verma P, Uniyal GC, Lal RK (2010) Growth kinetics and ginsenosides production in transformed hairy roots of American ginseng—Panax quinquefolium L. Biotechnol Lett 32:457–461
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87:9–19
Prasad A, Mathur A, Kalra A, Gupta MM, Lal RK, Mathur AK (2013) Fungal elicitor-mediated enhancement in growth and asiaticoside content of Centella asiatica L. shoot cultures. Plant Growth Regul 69:265–273
Qianliang M, Su C, Zheng C, Jia M, Zhang Q, Zhang H, Rahman K, Han T, Qin L (2013) Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J Exp Bot 64:5687–5694
Rahimi S, Kim YJ, Devi BSR, Oh JY, Kim SY, Kwon WS, Yang DC (2015a) Sodium nitroprusside enhances the elicitation power of methyl jasmonate for ginsenoside production in Panax ginseng roots. Res Chem Intermed. doi:10.1007/s11164-015-2188-x
Rahimi S, Kim YJ, Yang DC (2015b) Production of ginseng saponins: elicitation strategy and signal transductions. Appl Microbiol Biotechnol 99:6987–6996
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Santamaria AR, Mulinacci N, Valletta A, Innocenti M, Pasqua G (2011) Effects of elicitors on the production of resveratrol and viniferins in cell cultures of Vitis vinifera L. cv Italian. J Agric Food Chem 59:9094–9101
Satdive RK, Fulzele DP, Eapen S (2007) Enhanced production of azadirachtin by hairy root cultures of Azadirachta indica A. Juss by elicitation and media optimization. J Biotechnol 128:281–289
Schmidt W, Ebel J (1987) Specific binding of a fungal glucan phytoalexin elicitor to membrane fractions from soybean Glycine max. Proc Natl Acad Sci USA 84:4117–41121
Singh RK, Chaudhary BD (1979) Biometrical Methods in Quantitative Genetic Analysis. Kalyani publishers, India, New Delhi
Tewari RK, Paek KY (2011) Salicylic acid induced nitric oxide and ROS generation stimulate ginsenoside accumulation in Panax ginseng roots. J Plant Growth Reg 30:396–404
Tewari RK, Kim S, Hahn EJ, Paek KY (2008) Involvement of nitric oxide-induced NADPH oxidase in adventitious root growth and antioxidant defense in Panax ginseng. Plant Biotechnol Rep 2:113–122
Thanh NT, Murthy HN, Yu KW, Hahn EJ, Paek KY (2005) Methyl jasmonate elicitation enhanced synthesis of ginsenoside by cell suspension cultures of Panax ginseng in a 5 L balloon type bubble bioreactor. Appl Microbiol Biotechnol 67:197–201
Vasconsuelo A, Boland R (2007) Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci 172:861–875
Verma P, Khan SA, Mathur AK, Shanker K, Kalra A (2014) Fungal endophytes enhanced the growth and production kinetics of Vinca minor hairy roots and cell suspensions grown in bioreactor. Plant Cell Tissue Organ Cult 118:257–268
Wang W, Zhang ZY, Zhang JJ (2005) Enhancemnt of ginsenoside biosynthesis in high density cultivation of Panax notoginseng cells by various strategies of methyl jasmonate elicitation. Appl Microbiol Biotechnol 67:752–758
Wang J, Gao W, Zhang J, Huang T, Wen T, Zhang L, Huang L (2011) Production of saponins and polysaccharides in the presence of lactalbumin hydrolysate in Panax quinquefolium L. cell cultures. Plant Growth Reg 63:217–223
Wang P, Yongjun W, Yun F, Qunfang L, Wei W, Chengshuai Y, Lei Z, Zhao G, Yue J, Yan X, Zhou Z (2015) Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab Eng 29:97–105
Wu J, Zhong JJ (1999) Production of ginseng and its bioactive components in plant cell culture: current technological and applied aspects. J Biotechnol 68:89–99
Xu X, Hu X, Neill SJ, Fang J, Cai W (2005) Fungal elicitor induces singlet oxygen generation, ethylene release and saponin synthesis in cultured cells of Panax ginseng CA Meyer. Plant Cell Physiol 46:947–954
Yoshikawa M, Keen NT, Wang MC (1983) A receptor on soybean membranes for a fungal elicitor of phytoalexin accumulation. Plant Physiol 73:497–506
Yu KW, Murthy HN, Jeong CS, Hahn EJ, Paek KY (2005) Organic germanium stimulates the growth of ginseng adventitious roots and ginsenoside production. Process Biochem 40:2959–2961
Zhang B, Zheng LP, Wang JW (2012) Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl Microbiol Biotechnol 93:455–466
Zhao J, Lawrence CD, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
The authors are grateful to the Director, CSIR-CIMAP, for providing the necessary infrastructure required for the present investigation. Tanya Biswas also acknowledges the University Grants Commission for the award of a Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tanya Biswas declares that she has no conflict of interest. Alok Kalra declares that he has no conflict of interest. A K Mathur declares that he has no conflict of interest. R K Lal declares that he has no conflict of interest. Manju Singh declares that she has no conflict of interest. Archana Mathur declares that she has no conflict of interest.
Funding
This study was partially funded by a CSIR network project- BSC0117, entitled “Plant microbe and soil interactions.”
Ethical compliance
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Biswas, T., Kalra, A., Mathur, A.K. et al. Elicitors’ influenced differential ginsenoside production and exudation into medium with concurrent Rg3/Rh2 panaxadiol induction in Panax quinquefolius cell suspensions. Appl Microbiol Biotechnol 100, 4909–4922 (2016). https://doi.org/10.1007/s00253-015-7264-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7264-z