Abstract
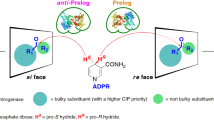
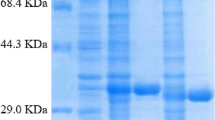
tert-Butyl (S)-6-chloro-5-hydroxy-3-oxohexanoate ((S)-CHOH) is a valuable chiral synthon, which is used for the synthesis of the cholesterol-lowering drugs atorvastatin and rosuvastatin. To date, only the alcohol dehydrogenases from Lactobacillus brevis (LbADH) and Lactobacillus kefir (LkADH) have demonstrated catalytic activity toward the asymmetric reduction of tert-butyl 6-chloro-3,5-dioxohexanoate (CDOH) to (S)-CHOH. Herein, a tetrad mutant of LkADH (LkTADH), A94T/F147L/L199H/A202L, was screened to be more efficient in this bioreduction process, exhibiting a 3.7- and 42-fold improvement in specific activity toward CDOH (1.27 U/mg) over LbADH (0.34 U/mg) and wild-type LkADH (0.03 U/mg), respectively. The molecular basis for the improved catalytic activity of LkTADH toward CDOH was investigated using homology modeling and docking analysis. Two major issues had a significant impact on the biocatalytic efficiency of this process, including (i) the poor aqueous stability of the substrate and (ii) partial substrate inhibition. A fed-batch strategy was successfully developed to address these issues and maintain a suitably low substrate concentration throughout the entire process. Several other parameters were also optimized, including the pH, temperature, NADP+ concentration and cell loading. A final CDOH concentration of 427 mM (100 g/L) gave (S)-CHOH in 94 % yield and 99.5 % e.e. after a reaction time of 38 h with whole cells expressing LkTADH. The space–time yield and turnover number of NADP+ in this process were 10.6 mmol/L/h and 16,060 mol/mol, respectively, which were the highest values ever reported. This new approach therefore represents a promising alternative for the efficient synthesis of (S)-CHOH.






Similar content being viewed by others
References
Amidjojo M, Franco-Lara E, Nowak A, Link H, Weuster-Botz D (2005) Asymmetric synthesis of tert-butyl (3R, 5S) 6-chloro-dihydroxyhexanoate with Lactobacillus kefir. Appl Microbiol Biotechnol 69:9–15
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485:185–194
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–252
Bradshaw CW, Hummel W, Wong CH (1992) Lactobacillus kefir alcohol dehydrogenase-a useful catalyst for synthesis. J Org Chem 57:1532–1536
Burton RL, Chen SW, Xu XL, Grant GA (2007) A novel mechanism for substrate inhibition in Mycobacterium tuberculosis D-3-phosphoglycerate dehydrogenase. J Biol Chem 282:31517–31524
Campopiano O, Mundorff E, Borup B, Voladri R (2011) Ketoreductase polypeptides for the production of azetidinone. US20110159567A1
Chen C, Joo JC, Brown G, Stolnikova E, Halavaty AS, Savchenko A, Anderson WF, Yakunin AF (2014) Structure-based mutational studies of substrate inhibition of betaine aldehyde dehydrogenase BetB from Staphylococcus aureus. Appl Environ Microbiol 80:3992–4002
Cleland WW (1979) Substrate inhibition. Methods Enzymol 63:500–513
Fenglai S (2007) Chemoenzymatic synthesis of key chiral intermediates of statins. Dissertation, Zhejiang University
Filling C, Berndt KD, Benach J, Knapp S, Prozorovski T, Nordling E, Oppermann U (2002) Critical residues for structure and catalysis in short-chain dehydrogenases/reductases. J Biol Chem 277:25677–25684
Gilbreath SG, Harris CM, Harris TM (1988) Biomimetic syntheses of pretetramides. 1. Synthesis of preteramide by tandem txtension of a polyketide chain. J Am Chem Soc 110:6172–6179
Godinho LF, Reis CR, van Merkerk R, Poelarends GJ, Quax WJ (2012) An esterase with superior activity and enantioselectivity towards 1,2-o-isopropylideneglycerol esters obtained by protein design. Adv Synth Catal 354:3009–3015
Gooding OW, Voladri R, Bautista A, Hopkins T, Huisman G, Jenne S, Ma S, Mundorff EC, Savile MM (2010) Development of a practical biocatalytic process for (R)-2-methylpentanol. Org Process Res Dev 14:119–126
Huang L, Ma H-M, Yu H-L, Xu J-H (2014) Altering the substrate specificity of reductase CgKR1 from Candida glabrata by protein engineering for bioreduction of aromatic α-keto esters. Adv Synth Catal 356:1943–1948
Huisman GW, Liang J, Krebber A (2010) Practical chiral alcohol manufacture using ketoreductases. Curr Opin Chem Biol 14:122–129
Hummel W, Riebel B (2000) Alcohol dehydrogenase and its use for the enzymatic production of chiral hydroxy compounds. US006037158A
Jackson B, Noti C, Hu GX (2012) Preparation of 3,5-dioxo hexanoate ester. WO2012130920A1
Kragl U, Niedermeyer U, Kula MR, Wandrey C (1990) Engineering aspects of enzyme engineering-continuous asymmetric C-C bond formation in an enzyme-menbrane-reactor. Ann N Y Acad Sci 613:167–175
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Leuchs S, Greiner L (2011) Alcohol dehydrogenase from Lactobacillus brevis: a versatile robust catalyst for enantioselective transformations. Chem Biochem Eng Q 25:267–281
Liang J, Mundorff E, Voladri R, Jenne S, Gilson L, Conway A, Krebber A, Wong J, Huisman G, Truesdell S, Lalonde J (2010) Highly enantioselective reduction of a small heterocyclic ketone: biocatalytic reduction of tetrahydrothiophene-3-one to the corresponding (R)-alcohol. Org Process Res Dev 14:188–192
LiCata VJ, Allewell NM (1997) Is substrate inhibition a consequence of allostery in aspartate transcarbamylase? Biophys Chem 64:225–234
Liljeblad A, Kallinen A, Kanerva LT (2009) Biocatalysis in the preparation of the statin side chain. Curr Org Synth 6:362–379
Maron DJ, Fazio S, Linton MF (2000) Current perspectives on statins. Circulation 101:207–213
Muller M (2005) Chemoenzymatic synthesis of building blocks for statin side chains. Angew Chem 44:362–365
Pace V, Cabrera AC, Ferrario V, Sinisterra JV, Ebert C, Gardossi L, Braiuca P, Alcántara AR (2011) Structural bases for understanding the stereoselectivity in ketone reductions with ADH from Thermus thermophilus: a quantitative model. J Mol Catal B Enzym 70:23–31
Patel JM (2009) Biocatalytic synthesis of atorvastatin intermediates. J Mol Catal B Enzym 61:123–128
Phosrithong N, Ungwitayatorn J (2010) Molecular docking study on anticancer activity of plant-derived natural products. Med Chem Res 19:817–835
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New York
Schlieben NH, Niefind K, Muller J, Riebel B, Hummel W, Schomburg D (2005) Atomic resolution structures of R-specific alcohol dehydrogenase from Lactobacillus brevis provide the structural bases of its substrate and cosubstrate specificity. J Mol Biol 349:801–813
Stein EA (2001) New statins and new doses of older statins. Curr Atheroscler Rep 3:14–18
Venkatachalam CM, Jiang X, Oldfield T, Waldman M (2003) LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol Graph Model 21:289–307
Villela Filho M, Stillger T, Müller M, Liese A, Wandrey C (2003) Is log P a convenient criterion to guide the choice of solvents for biphasic enzymatic reactions? Angew Chem 115:3101–3104
Wang LJ, Li CX, Ni Y, Zhang J, Liu X, Xu JH (2011) Highly efficient synthesis of chiral alcohols with a novel NADH-dependent reductase from Streptomyces coelicolor. Bioresour Technol 102:7023–7028
Weckbecker A, Hummel W (2006) Cloning, expression, and characterization of an (R)-specific alcohol dehydrogenase from Lactobacillus kefir. Biocatal Biotransfor 24:380–389
Westerbeek A, Szymanski W, Wijma HJ, Marrink SJ, Feringa BL, Janssen DB (2011) Kinetic resolution of alpha-bromoamides: experimental and theoretical investigation of highly enantioselective reactions catalyzed by haloalkane dehalogenases. Adv Synth Catal 353:931–944
Wolberg M, Hummel W, Wandrey C, Müller M (2000) Highly regio- and enantioselective reduction of 3,5-dioxocarboxylates. Angew Chem Int Ed 39:4306–4308
Wolberg M, Hummel W, Müller M (2001) Biocatalytic reduction of β, δ-diketo esters: a highly stereoselective approach to all four stereoisomers of a chlorinated β, δ-dihydroxy hexanoate. Chem Eur J 7:4562–4571
Wolberg M, Kaluzna IA, Müller M, Stewart JD (2004) Regio and enantioselective reduction of t-butyl 6-chloro-3,5-dioxohexanoate with baker’s yeast. Tetrahedron Asymmetry 15:2825–2828
Wolberg M, Filho MV, Bode S, Geilenkirchen P, Felsmann R, Liese A, Hummel W, Müller M (2008) Chemoenzymatic synthesis of the chiral side-chain of statins: application of an alcohol dehydrogenase catalysed ketone reduction on a large scale. Bioprocess Biosyst Eng 31:183–191
Ziegler J, Brandt W, Geissler R, Facchini PJ (2009) Removal of substrate inhibition and increase in maximal velocity in the short chain dehydrogenase/reductase salutaridine reductase involved in morphine biosynthesis. J Biol Chem 284:26758–26767
Acknowledgments
This work was financially supported by grants from the Key Project of Chinese National Programs for Fundamental Research and Development (no. 2011CB710800), the Hi-Tech Research and Development Program of China (no. 2011AA02A209), and the National Natural Science Foundation of China (no. 21476199).
Conflict of interest
The authors all declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1278 kb)
Rights and permissions
About this article
Cite this article
He, XJ., Chen, SY., Wu, JP. et al. Highly efficient enzymatic synthesis of tert-butyl (S)-6-chloro-5-hydroxy-3-oxohexanoate with a mutant alcohol dehydrogenase of Lactobacillus kefir . Appl Microbiol Biotechnol 99, 8963–8975 (2015). https://doi.org/10.1007/s00253-015-6675-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6675-1