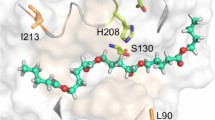
Abstract
Cutinases (EC 3.1.1.74) are extracellular enzymes that belong to α/β hydrolases. They are serine esterases with the classical Ser-His-Asp triad similar to several lipases and serine proteases. In nature, cutinases catalyse the hydrolysis of the polyesters of the cuticle and the suberin layers, which protect plant surfaces. Cutinase production is typical for plant pathogenic fungi, but also, bacterial cutinases and cutinases from plant pollen have been discovered. Cutinases are promiscuous esterases catalysing reactions with a wide range of different substrates, such as short-chain soluble esters, water-insoluble medium and long-chain triacylglycerols, polyesters and waxes. In the current work, an overview is given on suggested applications of cutinases in the textile industry, in laundry detergents, in processing of biomass and food, in biocatalysis and in detoxification of environmental pollutants. The applications are discussed from the point of view of cutinase properties—which properties of cutinases are already advantageous and which would be desired. In addition, improvements that have been made on cutinase performance by protein and reaction engineering are reviewed.






Similar content being viewed by others
References
Adams C, Miasnikov A (2010) Fungal cutinase from Magnaporthe griesea. WO2010107560
Agrawal PB (2005) The performance of cutinase and pectinase in cotton scouring. University of Twente
Agrawal PB, Nierstrasz VA, Warmoeskerken MMCG (2008) Role of mechanical action in low-temperature cotton scouring with F. solani pisi cutinase and pectate lyase. Enzym Microb Technol 42:473–482. doi:10.1016/j.enzmictec.2008.01.016
Agrawal PB, Nierstrasz VA, Warmoeskerken MMCG (2010) Ultrasound-boosted enzymatic cotton scouring process with cutinase and pectate lyase. Biocatal Biotransform 28:320–328. doi:10.3109/10242422.2010.528832
Ahn J-Y, Kim Y-H, Min J, Lee J (2006) Accelerated degradation of dipentyl phthalate by Fusarium oxysporum f. sp. pisi cutinase and toxicity evaluation of its degradation products using bioluminescent bacteria. Curr Microbiol 52:340–344. doi:10.1007/s00284-005-0124-9
Alisch-Mark M, Herrmann A, Zimmermann W (2006) Increase of the hydrophilicity of polyethylene terephthalate fibres by hydrolases from Thermomonospora fusca and Fusarium solani f. sp. pisi. Biotechnol Lett 28:681–685. doi:10.1007/s10529-006-9041-7
Andersen KE, Borch K, Krebs LNE, Steffen E, Landvik S, Schnorr KM (2006) Plant extraction process. WO2006111163
Araújo R, Silva C, O’Neill A, Micaelo N, Guebitz G, Soares CM, Casal M, Cavaco-Paulo A (2007) Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J Biotechnol 128:849–857. doi:10.1016/j.jbiotec.2006.12.028
Badenes SM, Lemos F, Cabral JMS (2010) Transesterification of oil mixtures catalyzed by microencapsulated cutinase in reversed micelles. Biotechnol Lett 32:399–403. doi:10.1007/s10529-009-0172-5
Badenes SM, Lemos F, Cabral JMS (2011a) Kinetics and mechanism of the cutinase-catalyzed transesterification of oils in AOT reversed micellar system. Bioprocess Biosyst Eng 34:1133–1142. doi:10.1007/s00449-011-0564-5
Badenes SM, Lemos F, Cabral JMS (2011b) Stability of cutinase, wild type and mutants, in AOT reversed micellar system-effect of mixture components of alkyl esters production. J Chem Technol Biotechnol 86:34–41. doi:10.1002/jctb.2505
Baker CJ, Bateman DF (1978) Cutin degradation by plant pathogenic fungi. Phytopathology 68:1577–1584
Balcão VM, Malcata FX (1998) Lipase catalyzed modification of milkfat. Biotechnol Adv 16:309–341. doi:10.1016/S0734-9750(97)00064-5
Bergé A, Cladière M, Gasperi J, Coursimault A, Tassin B, Moilleron R (2013) Meta-analysis of environmental contamination by phthalates. Environ Sci Pollut Res Int 20:8057–8076. doi:10.1007/s11356-013-1982-5
Berk Z (1976) The biochemistry of foods. Elsevier, Amsterdam
Boom A, Sinninge Damsté JS, de Leeuw JW (2005) Cutan, a common aliphatic biopolymer in cuticles of drought-adapted plants. Org Geochem 36:595–601. doi:10.1016/j.orggeochem.2004.10.017
Brissos V, Eggert T, Cabral JMS, Jaeger K-E (2008a) Improving activity and stability of cutinase towards the anionic detergent AOT by complete saturation mutagenesis. Protein Eng Des Sel 21:387–393. doi:10.1093/protein/gzn014
Brissos V, Melo EP, Martinho JMG, Cabral JMS (2008b) Biochemical and structural characterisation of cutinase mutants in the presence of the anionic surfactant AOT. Biochim Biophys Acta 1784:1326–1334. doi:10.1016/j.bbapap.2008.04.017
Carneiro F, Silva C, Matamá T, Araújo R, Casal M, Güebitz G, Cavaco-Paulo A (2005) Method for the modification of polyacrylonitrile fibres containing vinyl acetate as a comonomer and polyamide fibres, using a cutinase enzyme. WO2005040487
Carvalho CML, Serralheiro MLM, Cabral JMS, Aires-Barros MR (1997) Application of factorial design to the study of transesterification reactions using cutinase in AOT-reversed micelles. Enzym Microb Technol 21:117–123. doi:10.1016/S0141-0229(96)00245-1
Carvalho CM, Aires-Barros MR, Cabral JM (1999) Cutinase: from molecular level to bioprocess development. Biotechnol Bioeng 66:17–34
Carvalho CML, Aires-Barros MR, Cabral JMS (2000) Kinetics of cutinase catalyzed transesterification in AOT reversed micelles: modeling of a batch stirred tank reactor. J Biotechnol 81:1–13. doi:10.1016/S0168-1656(00)00260-1
Chen S, Tong X, Woodard RW, Du G, Wu J, Chen J (2008) Identification and characterization of bacterial cutinase. J Biol Chem 283:25854–25862. doi:10.1074/jbc.M800848200
Chen S, Su L, Chen J, Wu J (2013) Cutinase: characteristics, preparation, and application. Biotechnol Adv 31:1754–1767. doi:10.1016/j.biotechadv.2013.09.005
Cunnah PJ, Aires-Barros MR, Cabral JMS (1996) Esterification and transesterification catalysed by cutinase in reverse micelles of CTAB for the synthesis of short chain esters. Biocatal Biotransform 14:125–146
De Barros DPC, Fonseca LP, Fernandes P, Cabral JMS, Mojovic L (2009) Biosynthesis of ethyl caproate and other short ethyl esters catalyzed by cutinase in organic solvent. J Mol Catal B Enzym 60:178–185. doi:10.1016/j.molcatb.2009.05.004
Degani O, Gepstein S, Dosoretz CG (2002) Potential use of cutinase in enzymatic scouring of cotton fiber cuticle. Appl Biochem Biotechnol 102–103:277–290. doi:10.1385/ABAB:102-103:1-6:277
Dutta K, Dasu VV (2011) Synthesis of short chain alkyl esters using cutinase from Burkholderia cepacia NRRL B2320. J Mol Catal B Enzym 72:150–156. doi:10.1016/j.molcatb.2011.05.013
Dutta K, Sen S, Veeranki VD (2009) Production, characterization and applications of microbial cutinases. Process Biochem 44:127–134. doi:10.1016/j.procbio.2008.09.008
Egmond MR, van Bemmel CJ (1997) Lipases, part A: biotechnology. Methods Enzymol 284:119–129. doi:10.1016/S0076-6879(97)84008-6
Ettinger WF, Thukral SK, Kolattukudy PE (1987) Structure of cutinase gene, cDNA, and the derived amino acid sequence from phytopathogenic fungi. Biochemistry 26:7883–7892. doi:10.1021/bi00398a052
Fett WF, Wijey C, Moreau RA, Osman SF (1999) Production of cutinase by Thermomonospora fusca ATCC 27730. J Appl Microbiol 86:561–568. doi:10.1046/j.1365-2672.1999.00690.x
Flipsen JAC, Appel ACM, van der Hijden HTWM, Verrips CT (1998) Mechanism of removal of immobilized triacylglycerol by lipolytic enzymes in a sequential laundry wash process. Enzym Microb Technol 23:274–280. doi:10.1016/S0141-0229(98)00050-7
Gandini A, Pascoal Neto C, Silvestre AJD (2006) Suberin: a promising renewable resource for novel macromolecular materials. Prog Polym Sci 31:878–892. doi:10.1016/j.progpolymsci.2006.07.004
Gonçalves APV, Cabral JMS, Aires-Barros MR (1996) Immobilization of a recombinant cutinase by entrapment and by covalent binding. Appl Biochem Biotechnol 60:217–228. doi:10.1007/BF02783585
Gonçalves AM, Schacht E, Matthijs G, Aires Barros MR, Cabral JMS, Gil MH (1999) Stability studies of a recombinant cutinase immobilized to dextran and derivatized silica supports. Enzym Microb Technol 24:60–66. doi:10.1016/S0141-0229(98)00089-1
Gonçalves AM, Serro AP, Aires-Barros MR, Cabral JMS (2000) Effects of ionic surfactants used in reversed micelles on cutinase activity and stability. Biochim Biophys Acta Prot Struct Mol Enzymol 1480:92–106. doi:10.1016/S0167-4838(00)00093-5
Graça J, Santos S (2007) Suberin: a biopolyester of plants’ skin. Macromol Biosci 7:128–135. doi:10.1002/mabi.200600218
Gross RA, Ganesh M, Lu W (2010) Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol 28:435–443. doi:10.1016/j.tibtech.2010.05.004
Guebitz GM, Cavaco-Paulo A (2008) Enzymes go big: surface hydrolysis and functionalization of synthetic polymers. Trends Biotechnol 26:32–38. doi:10.1016/j.tibtech.2007.10.003
Hardin IR, Li Y, Akin D (1998) Cotton wall structure and enzymatic treatments. In: Eriksson KE, Cavaco-Paulo A (eds) Enzyme applications in fiber processing. ACS Symposium Series 687, Washington, DC, pp 190–203
Herrero Acero E, Ribitsch D, Dellacher A, Zitzenbacher S, Marold A, Steinkellner G, Gruber K, Schwab H, Guebitz GM (2013) Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol Bioeng 110:2581–2590. doi:10.1002/bit.24930
Hijden HTWM, Marugg J, Warr JF, Klugkist J, Musters W, Hondmann DH (1994) Enzymatic detergent compositions. WO9403578
Horii K, Adachi T, Tanino T, Tanaka T, Kotaka A, Sahara H, Hashimoto T, Kuratani N, Shibasaki S, Ogino C, Noda H, Hata Y, Ueda M, Kondo A (2010) Fatty acid production from butter using novel cutinase-displaying yeast. Enzym Microb Technol 46:194–199. doi:10.1016/j.enzmictec.2009.10.008
Hunsen M, Azim A, Mang H, Wallner SR, Ronkvist A, Xie W, Gross RA (2007) A cutinase with polyester synthesis activity. Macromolecules 40:148–150. doi:10.1021/ma062095g
Iversen T, Nilsson H, Olsson A (2010) A method for separating from suberin and/or cutin containing plants, a solid and/or oil fraction enriched in cis-9,10-epoxy-18-hydroxyoctadecanoic acid. WO2010093320
Järvinen R, Silvestre AJD, Holopainen U, Kaimainen M, Nyyssölä A, Gil AM, Pascoal Neto C, Lehtinen P, Buchert J, Kallio H (2009) Suberin of potato (Solanum tuberosum var. Nikola): comparison of the effect of cutinase CcCut1 with chemical depolymerization. J Agric Food Chem 57:9016–9027
Kerstiens G (1996) Cuticular water permeability and its physiological significance. J Exp Bot 47:1813–1832. doi:10.1093/jxb/47.12.1813
Kim Y-H, Lee J, Ahn J-Y, Gu MB, Moon S-H (2002) Enhanced degradation of an endocrine-disrupting chemical, butyl benzyl phthalate, by Fusarium oxysporum f. sp. pisi cutinase. Appl Environ Microbiol 68:4684–4688
Kim Y-H, Lee J, Moon S-H (2003) Degradation of an endocrine disrupting chemical, DEHP [di-(2-ethylhexyl)-phthalate], by Fusarium oxysporum f. sp. pisi cutinase. Appl Microbiol Biotechnol 63:75–80. doi:10.1007/s00253-003-1332-5
Kim Y-H, Ahn J-Y, Moon S-H, Lee J (2005) Biodegradation and detoxification of organophosphate insecticide, malathion by Fusarium oxysporum f. sp. pisi cutinase. Chemosphere 60:1349–1355. doi:10.1016/j.chemosphere.2005.02.023
Koeller W, Kolattukudy PE (1982) Mechanism of action of cutinase: chemical modification of the catalytic triad characteristic for serine hydrolases. Biochemistry 21:3083–3090. doi:10.1021/bi00256a008
Kolattukudy PE (1980) Biopolyester membranes of plants: cutin and suberin. Science 208:990–1000. doi:10.1126/science.208.4447.990
Kolattukudy PE (1984) Cutinases from fungi and pollen. In: Borgström B, Brockman H (eds) Lipases vol. C. Elsevier, Amsterdam, pp 471–504
Kolattukudy PE (2001) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71:1–49
Kolattukudy PE, Poulose A (1991) Cutinase cleaning compositions. US4981611
Kontkanen H, Westerholm-Parvinen A, Saloheimo M, Bailey M, Rättö M, Mattila I, Mohsina M, Kalkkinen N, Nakari-Setälä T, Buchert J (2009) Novel Coprinopsis cinerea polyesterase that hydrolyzes cutin and suberin. Appl Environ Microbiol 75:2148–2157. doi:10.1128/AEM.02103-08
Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42:51–80
Lee SH, Song WS, Kim HR (2010) Cutinase treatment of cotton fabrics. Fibers Polym 10:802–806. doi:10.1007/s12221-009-0802-5
Li D, Ashby AM, Johnstone K (2003) Molecular evidence that the extracellular cutinase Pbc1 is required for pathogenicity of Pyrenopeziza brassicae on oilseed rape. Mol Plant Microbe Interact 16:545–552. doi:10.1094/MPMI.2003.16.6.545
Longhi S, Cambillau C (1999) Structure-activity of cutinase, a small lipolytic enzyme. Biochim Biophys Acta Mol Cell Biol Lipids 1441:185–196. doi:10.1016/S1388-1981(99)00159-6
Longhi S, Czjzek M, Lamzin V, Nicolas A, Cambillau C (1997) Atomic resolution (1.0 A) crystal structure of Fusarium solani cutinase: stereochemical analysis. J Mol Biol 268:779–799. doi:10.1006/jmbi.1997.1000
Mannesse MLM, Cox RC, Koops BC, Verheij HM, de Haas GH, Egmond MR, van der Hijden HTWM, de Vlieg J (1995) Cutinase from Fusarium solani pisi hydrolyzing triglyceride analogs. Effect of acyl chain length and position in the substrate molecule on activity and enantioselectivity. Biochemistry 34:6400–6407. doi:10.1021/bi00019a020
Martinez C, Nicolas A, van Tilbeurgh H, Egloff MP, Cudrey C, Verger R, Cambillau C (1994) Cutinase, a lipolytic enzyme with a preformed oxyanion hole. Biochemistry 33:83–89. doi:10.1021/bi00167a011
Matamá T, Vaz F, Gübitz GM, Cavaco-Paulo A (2006) The effect of additives and mechanical agitation in surface modification of acrylic fibres by cutinase and esterase. Biotechnol J 1:842–849. doi:10.1002/biot.200600034
Metzger JO, Bornscheuer U (2006) Lipids as renewable resources: current state of chemical and biotechnological conversion and diversification. Appl Microbiol Biotechnol 71:13–22. doi:10.1007/s00253-006-0335-4
Nimchua T, Punnapayak H, Zimmermann W (2007) Comparison of the hydrolysis of polyethylene terephthalate fibers by a hydrolase from Fusarium oxysporum LCH I and Fusarium solani f. sp. pisi. Biotechnol J 2:361–364. doi:10.1002/biot.200600095
Nyyssölä A, Pihlajaniemi V, Järvinen R, Mikander S, Kontkanen H, Kruus K, Kallio H, Buchert J (2013) Screening of microbes for novel acidic cutinases and cloning and expression of an acidic cutinase from Aspergillus niger CBS 513.88. Enzym Microb Technol 52:272–278
Nyyssölä A, Pihlajaniemi V, Häkkinen M, Kontkanen H, Saloheimo M, Nakari-Setälä T (2014) Cloning and characterization of a novel acidic cutinase from Sirococcus conigenus. Appl Microbiol Biotechnol 98:3639–3650. doi:10.1007/s00253-013-5293-z
Oeser T, Wei R, Baumgarten T, Billig S, Föllner C, Zimmermann W (2010) High level expression of a hydrophobic poly(ethylene terephthalate)-hydrolyzing carboxylesterase from Thermobifida fusca KW3 in Escherichia coli BL21(DE3). J Biotechnol 146:100–104. doi:10.1016/j.jbiotec.2010.02.006
Papadimitriou V, Xenakis A, Cazianis CT, Kolisis FN (1997) Structural and catalytic aspects of cutinase in w/o microemulsions. Colloid Polym Sci 275:609–616. doi:10.1007/s003960050126
Pio TF, Macedo GA (2009) Cutinases: properties and industrial applications. Adv Appl Microbiol 66:77–95. doi:10.1016/S0065-2164(08)00804-6
Pollard M, Beisson F, Li Y, Ohlrogge JB (2008) Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13:236–246. doi:10.1016/j.tplants.2008.03.003
Poulose A, Boston M (1994) Enzyme assisted degradation of surface membranes of harvested fruits and vegetables. US5298265
Regado MA, Cristóvão BM, Moutinho CG, Balcão VM, Aires-Barros R, Ferreira JPM, Xavier Malcata F (2007) Flavour development via lipolysis of milkfats: changes in free fatty acid pool. Int J Food Sci Technol 42:961–968. doi:10.1111/j.1365-2621.2006.01317.x
Ribitsch D, Yebra AO, Zitzenbacher S, Wu J, Nowitsch S, Steinkellner G, Greimel K, Doliska A, Oberdorfer G, Gruber CC, Gruber K, Schwab H, Stana-Kleinschek K, Acero EH, Guebitz GM (2013) Fusion of binding domains to Thermobifida cellulosilytica cutinase to tune sorption characteristics and enhancing PET hydrolysis. Biomacromolecules 14:1769–1776. doi:10.1021/bm400140u
Roussel A, Amara S, Nyyssölä A, Mateos-Diaz E, Blangy S, Kontkanen H, Westerholm-Parvinen A, Carrière F, Cambillau C (2014) A cutinase from Trichoderma reesei with a lid-covered active site and kinetic properties of true lipases. J Mol Biol. doi:10.1016/j.jmb.2014.09.003
Sebastião MJ, Cabral JM, Aires-Barros MR (1993) Synthesis of fatty acid esters by a recombinant cutinase in reversed micelles. Biotechnol Bioeng 42:326–332. doi:10.1002/bit.260420309
Silva C, Matamá T, Guebitz GM, Cavaco-Paulo A (2005a) Influence of organic solvents on cutinase stability and accessibility to polyamide fibers. J Polym Sci A Polym Chem 43:2749–2753. doi:10.1002/pola.20739
Silva CM, Carneiro F, O’Neill A, Fonseca LP, Cabral JSM, Guebitz G, Cavaco-Paulo A (2005b) Cutinase—a new tool for biomodification of synthetic fibers. J Polym Sci A Polym Chem 43:2448–2450. doi:10.1002/pola.20684
Silva C, Araújo R, Casal M, Gübitz GM, Cavaco-Paulo A (2007) Influence of mechanical agitation on cutinases and protease activity towards polyamide substrates. Enzym Microb Technol 40:1678–1685. doi:10.1016/j.enzmictec.2006.09.001
Silva C, Da S, Silva N, Matamá T, Araújo R, Martins M, Chen S, Chen J, Wu J, Casal M, Cavaco-Paulo A (2011) Engineered Thermobifida fusca cutinase with increased activity on polyester substrates. Biotechnol J 6:1230–1239. doi:10.1002/biot.201000391
Stavila E, Alberda van Ekenstein GOR, Loos K (2013a) Enzyme-catalyzed synthesis of aliphatic-aromatic oligoamides. Biomacromolecules 14:1600–1606. doi:10.1021/bm400243a
Stavila E, Arsyi RZ, Petrovic DM, Loos K (2013b) Fusarium solani pisi cutinase-catalyzed synthesis of polyamides. Eur Polym J 49:834–842. doi:10.1016/j.eurpolymj.2012.12.010
Tavanai H (2009) A new look at the modification of polyethylene terephthalate by sodium hydroxide. J Text Inst 100:633–639
Ternström T, Svendsen A, Akke M, Adlercreutz P (2005) Unfolding and inactivation of cutinases by AOT and guanidine hydrochloride. Biochim Biophys Acta 1748:74–83. doi:10.1016/j.bbapap.2004.12.014
Viksoe-Nielsen A, Soerensen BH (2009a) Cutinase for detoxification of feed products. WO2009080701
Viksoe-Nielsen A, Soerensen BH (2009b) Detoxification of aflatoxin in feed products. US2009226570
Xun H, Chen Z, Guangming Z, Danlian H, Liang L, Cui L, Meihua Z, Chao H, Ningjie L, Zhen W, Piao X, Min C (2014) Immobilized cutinase and preparation method and application thereof in removal of phthalic acid esters in water. CN103756991
Yan HJ, Du GC, Chen J (2011) Enhancement of cotton waxes removal with Thermobifida fusca cutinase by temperature control process. Adv Mater Res 81–86
Yang S, Xu H, Yan Q, Liu Y, Zhou P, Jiang Z (2013) A low molecular mass cutinase of Thielavia terrestris efficiently hydrolyzes poly(esters). J Ind Microbiol Biotechnol 40:217–226. doi:10.1007/s10295-012-1222-x
Zhang Y, Chen S, Xu M, Cavaco-Paulo A, Cavoco-Paulo A, Wu J, Chen J (2010) Characterization of Thermobifida fusca cutinase-carbohydrate-binding module fusion proteins and their potential application in bioscouring. Appl Environ Microbiol 76:6870–6876. doi:10.1128/AEM.00896-10
Zoungrana T, Findenegg GH, Norde W (1997) Structure, stability, and activity of adsorbed enzymes. J Colloid Interface Sci 190:437–448. doi:10.1006/jcis.1997.4895
Acknowledgments
I thank Ossi Turunen, Ville Pihlajaniemi and Mika Sipponen for help with the artwork.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nyyssölä, A. Which properties of cutinases are important for applications?. Appl Microbiol Biotechnol 99, 4931–4942 (2015). https://doi.org/10.1007/s00253-015-6596-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6596-z