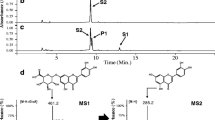
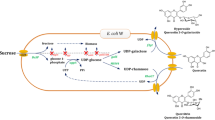
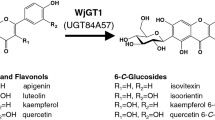
Abstract
Flavonoids are plant secondary metabolites containing several hydroxyl groups that are targets for modification reactions such as methylation and glycosylation. In plants, flavonoids are present as glycones. Although glucose is the most common sugar attached to flavonoids, arabinose, galactose, glucuronic acid, rhamnose, and xylose are also linked to flavonoids. Depending on the kind and the position of the attached sugar, flavonoid glycones show different biological properties. Flavonoid-O-glycosides are synthesized by uridine diphosphate-dependent glycosyltransferases (UGTs), which use nucleotide sugar as a sugar donor and a diverse compound as a sugar acceptor. Recently, diverse flavonoid-O-glycosides have been synthesized in Escherichia coli by introducing UGTs from plants and bacteria and modifying endogenous pathways. The nucleotide sugar biosynthesis pathway in E. coli has been engineered to provide the proper nucleotide sugar for flavonoid-O-glycoside biosynthesis. In this review, we will discuss recent advances in flavonoid-O-glycoside biosynthesis using engineered E. coli.

Similar content being viewed by others
References
Ahn BC, Kim BG, Jeon YM, Lee EJ, Lim Y, Ahn J-H (2009) Formation of flavone di-O-glucosides using a glycosyltransferase from Bacillus cereus. J Microbiol Biotechnol 19:387–390
Bhan N, Xu P, Koffas MA (2013) Pathway and protein engineering approaches to produce novel and commodity small molecules. Curr Opin Biotechnol 24:1137–1143
Bowles D, Lim E-K, Poppenberger B, Vaistij FE (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57:567–597
Brazier-Hicks M, Evans KM, Gershater MC, Puschmann H, Steel PG, Edwards R (2009) The C-glycosylation of flavonoids in cereals. J Biol Chem 284:17926–17934
Breazeale SD, Riberio AA, McClerren AL, Raetz CRH (2005) Aformyltransferase required for polymyxin resistance in Escherichia coli and the modification of lipid A with 4-amino-4-deoxy-L-arabinose. J Biol Chem 280:14154–14167
Burget EG, Verma R, Mølhøj M, Reiter W-D (2003) The biosynthesis of L-arabinose in plants: molecular cloning and characterization of a Golgi-localized UDP-D-xylose 4-epimerase encoded by the MUR4 gene of Arabidopsis. Plant Cell 15:523–553
Caputi L, Lim E-K, Bowles DJ (2008) Discovery of new biocatalysts for the glycosylation of terpenoid scaffolds. Chem Eur J 14:6656–6662
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Dong C, Beis K, Giraud MF, Blankenfeldt W, Allard S, Major LL, Kerr ID, Whitfield C, Naismith JH (2003) A structural perspective on the enzymes that convert dTDP-D-glucose into dTDP-L-rhamnose. Biochem Soc Trans 31:532–536
Du J, Shao Z, Zhao H (2011) Engineering microbial factories for synthesis of value-added products. J Ind Microbiol Biotechnol 38:873–890
Gu X, Lee SG, Bar-Peled M (2011) Biosynthesis of UDP-xylose and UDP-arabinose in Sinorhizobium meliloti 1021: first characterization of a bacterial UDP-xylose synthase, and UDP-xylose 4-epimerase. Microbiology 157:260–269
Han SH, Kim B-G, Yoon JA, Chong Y, Ahn J-H (2014) Synthesis of flavonoid O-pentosides by Escherichia coli through engineering nucleotide sugar synthesis pathway and glycosyltransferase. Appl Environ Microbiol 80:2754–2762
Hartmann T (2007) From waste products to ecochemicals: fifty years research of plant secondary metabolism. Phytochemistry 68:2831–2846
He X-Z, Li W-S, Blount JW, Dixon RA (2008) Regioselective synthesis of plant (iso)flavone glycosides in Escherichia coli. Appl Microbiol Biotechnol 80:253–260
Jones P, Messner B, Nakajima J-I, Schäffner AR, Saito K (2003) UGT73C6 and UGT78D1, glycosyltransferases involved in flavonol glycoside biosynthesis in Arabidopsis thaliana. J Biol Chem 278:43910–43918
Jüergenliemk G, Boje K, Huewel S, Lohmann C, Galla HJ, Nahrstedt A (2003) In vitro studies indicate that miquelianin (quercetin 3-O-beta-D-glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med 69:1013–1017
Kim JH, Kim BG, Kim JA, Park Y, Lee YJ, Lim Y, Ahn J-H (2007) Glucosylation of flavonoids with E. coli expressing glycosyltransferase from Xanthomonas campestris. J Microbiol Biotechnol 17:539–542
Kim JH, Shin KH, Ko JH, AHn J-H (2006) GLucosylation of flavonols by Escherichia coli expressiong glucosyltransferase from rice (Oryza sativa). J Biosci Bioeng 102:135–137
Kim B-G, Jung NR, Joe EJ, Hur H-G, Lim Y, Chong Y, Ahn J-H (2010a) Bacterial synthesis of a flavonoid deoxyaminosugar conjugate in Escherichia coli expressing a glycosyltransferase of Arabidopsis thaliana. Chem Biol Chem 11:2389–2392
Kim B-G, Sung SH, Jung NR, Chong Y, Ahn J-H (2010b) Biological synthesis of isorhamnetin 3-O-glucoside using engineered glucosyltransferase. J Mol Catal B-Enzym 63:194–199
Kim BG, Kim HJ, Ahn J-H (2012a) Production of bioactive flavonol rhamnosides by expression of plant genes in Escherichia coli. J Agric Food Chem 60:11143–11148
Kim B-G, Sung SH, Ahn J-H (2012b) Biological synthesis of quercetin 3-O-N-acetylglucosamine conjugate using engineered Escherichia coli expressing UGT78D2. Appl Microbiol Biotechnol 93:2447–2453
Kim HJ, Kim BG, Ahn J-H (2013a) Regioselective synthesis of flavonoid bisglycosides using Escherichia coli harboring two glycosyltransferases. Appl Microbiol Biotechnol 97:5275–5282
Kim MJ, Kim B-G, Ahn J-H (2013b) Biosynthesis of bioactive O-methylated flavonoids in Escherichia coli. Appl Microbiol Biotechnol 97:7195–7720
Kim SY, Lee HR, Park K-s, Kim BG, Ahn J-H (2015) Metabolic engineering of Escherichia coli for the biosynthesis of flavonoid O-glucuronides and flavonoid O-galactoside. Appl Microbiol Biotechnol 99:2233–2242
Ko JH, Kim BG, Ahn J-H (2006) Glycosylation of flavonoids with a glycosyltransferase from Bacillus cereus. FEMS Microbiol Lett 258:263–268
Koirala N, Pandey RP, Thang DV, Jung HJ, Sohng JK (2014) Glycosylation and subsequent malonylation of isoflavonoids in E. coli: strain development, production and insights into future metabolic perspectives. J Ind Microbiol Biotechnol 41:1647–1658
Kumar S, Pandey AK (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:162750
Levander F, Svensson M, Rädström P (2002) Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophiles. Appl Environ Microbiol 68:784–790
Lim E-K, Ashford DA, Hou B, Jackson RG, Bowles DJ (2004) Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetinglucosides. Biotechnol Bioeng 87:623–631
Malla S, Koffas MAG, Kazlauskas R, Kim B-G (2012) Production of 7-O-methyl aromadendrin, a medicinally valuable flavonoid, in Escherichia coli. Appl Environ Microbiol 78:684–694
Malla S, Pandey RP, Kim B-G, Sohng JK (2013) Regiospecific modifications of naringenin for astragalin production in Escherichia coli. Biotechnol Bioeng 110:2525–2535
Mao Z, Shin H-D, Chen RR (2006) Engineering the E. coli UDP-glucose synthesis pathway for oligosaccharide synthesis. Biotechnol Prog 22:369–374
Miller KD, Guyon V, Evans JN, Shuttleworth WA, Taylor LP (1999) Purification cloning and heterologous expression of a catalytically efficient flavonol 3-O-galactosyltransferase expressed in the male gametophyte of Petunia hybrid. J Biol Chem 274:34011–34019
Miyahisa I, Funa N, Ohnishi Y, Martens S, Moriguchi T, Horinouchi S (2006) Combinatorial biosynthesis of flavones and flavonols in Escherichia coli. Appl Microbiol Biotechnol 71:53–58
Noguchi A, Horikawa M, Fukui Y, Fukuchi-Mizutani M, Luchi-Okada A, Ishiguro M, Kiso Y, Nakayama T, Ono E (2009) Local differentiation of sugar donor specificity of flavonoid glycosyltransferase in Lamiales. Plant Cell 21:1556–1572
Oh T-J, Mo SJ, Yoon YJ, Sohng JK (2007) Discovery and molecular engineering of sugar-containing natural product biosynthetic pathways in actinomycetes. J Microbiol Biotechnol 17:1909–1921
Oka T, Nemoto T, Jigami Y (2007) Functional analysis of Arabidopsis thaliana RHM2/MUM4, a multidomain protein involved in UDP-D-glucose to UDP-L-rhamnose conversion. J Biol Chem 282:5389–5403
Ono E, Homma Y, Horikawa M, Kunikane-Doi S, Imai H, Takahashi S, Kawai Y, Ishiguro M, Fukui Y, Nakayama T (2010) Functional differentiation of the glycosyltransferases that contribute to the chemical diversity of bioactive flavonol glycosides in grapevines (Vitis vinifera). Plant Cell 22:2856–2871
Osmani SA, Bak S, Møller BL (2009) Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry 70:325–347
Pandey RP, Malla S, Simkhada B, Kim B-G, Sohng JG (2013) Production of 3-O-xylosyl quercetin in Escherichia coli. Appl Microbiol Biotechnol 97:1889–1901
Pandey RP, Gurung RB, Parajuli P, Koirala N, Tuoi LT, Sohng JK (2014) Assessing acceptor substrate promiscuity of YjiC-mediated glycosylation toward flavonoids. Carbohydr Res 393:26–31
Reiter W-D (2008) Biochemical genetics of nucleotide sugar interconversion reactions. Curr Opin Plant Biol 11:236–243
Roepke J, Bozzo GG (2013) Biocatalytic synthesis of quercetin 3-O-glucoside-7-O-rhamnoside by metabolic engineering of Escherichia coli. Chem Biol Chem 14:2418–2422
Santos CNS, Koffas M, Stephanopoulos (2011) Optimization of a heterologous pathway for the production of flavonoids from glucose. Metab Eng 13:392–400
Scheffers D-J, Pinho MG (2005) Bacterial cell wall synthesis: new insights from localization studies. Microbiol Mol Biol Rev 69:585–607
Schwab W, Fischer TC, Giri A, Wüst M (2015) Potential applications of glucosyltransferases in terpene glucosideproduction: impacts on the use of aroma and fragrance. Appl Microbiol Biotechnol 99:165–174
Seifert GJ (2004) Nucleotide sugar interconversion and cell wall biosynthesis: how to bring the inside to the outside. Curr Opin Plant Biol 7:277–284
Simkhada D, Lee HC, Sohng JK (2010) Genetic engineering approach for the production of rhamnosyl and allosylflavonoids from Escherichia coli. Biotechnol Bioeng 107:154–162
Soundararajan R, Wishart AD, Rupasinghe HP, Arcellana-Panlilio M, Nelson CM, Mayne M, Robertson GS (2008) Quercetin 3-glucoside protects neuroblastoma (SH-SY5Y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis. J Biol Chem 283:2231–2245
Thibodeaux CJ, Melanҫon C, H-w L (2007) Unusual sugar biosynthesis and natural product glycodiversification. Nature 446:1008–1016
Thuan NH, Pandey RP, Thuy TT, Park JW, Sohng JK (2013) Improvement of regio-specific production of myricetin-3-O-α-L-rhamnoside in engineered Escherichia coli. Appl Biochem Biotechnol 171:1956–1967
Veitch NC, Grayer RJ (2011) Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep 28:1626–1695
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3:2–20
Vogt T, Jones P (2000) Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trend Plant Sci 5:380–386
Wang X (2009) Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett 583:3303–3309
Wang L, Han W, Xie C, Hou J, Fang Q, Gu J, Wang PG, Cheng J (2013) Comparing the acceptor promiscuity of a Rosa hybrid glucosyltransferase RhGT1 and an engineered microbial glucosyltransferase OleDPSA toward a small flavonoid library. Carbohydr Res 368:73–77
Watts KT, Lee PC, Schmidt-Dannert C (2004) Exploring recombinant flavonoid biosynthesis in metabolically engineered Escherichia coli. Chem Biol Chem 5:500–507
Willits MG, Giovanni M, Prata RT, Kramer CM, De Luca V, Steffens JC, Graser G (2004) Bio-fermentation of modified flavonoids: an example of in vivo diversification of secondary metabolites. Phytochemistry 5:31–41
Winkel-Shirely B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Yan Y, Li Z, Koffas MAG (2007) High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol Bioeng 100:126–140
Yang S-M, Han SH, Kim B-G, Ahn J-H (2014) Production of kaempferol 3-O-rhamnoside from glucose using engineered Escherichia coli. J Ind Microbiol Biotechnol 41:1311–1318
Yonekura-Sakakibara K, Hanada K (2011) An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J 66:182–193
Yonekura-Sakakibara K, Tohge T, Matsuda F, Nakabayashi R, Takayama H, Niida R, Watanabe-Takahashi A, Inoue E, Saito K (2008) Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene-metabolite correlation in Arabidopsis. Plant Cell 20:2160–2176
Yonekura-Sakakibara K, Fukushima A, Nakabayashi R, Hanada K, Matsuda F, Sugawara S, Inoue E, Kuromori T, Ito T, Shinozaki K, Wangwattana B, Yamazaki M, Saito K (2012) Two glycosyltransferases involved in anthocyanin modification delineated by transcriptome independent component analysis in Arabidopsis thaliana. Plant J 69:154–167
Yoon J-A, Kim B-G, Lee WJ, Lim Y, Chong Y, Ahn J-H (2012) Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl Environ Microbiol 78:4256–4262
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (PJ01114801), Rural Development Administration, and Priority Research Centers Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology 2009-0093824).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, B.G., Yang, S.M., Kim, S.Y. et al. Biosynthesis and production of glycosylated flavonoids in Escherichia coli: current state and perspectives. Appl Microbiol Biotechnol 99, 2979–2988 (2015). https://doi.org/10.1007/s00253-015-6504-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6504-6