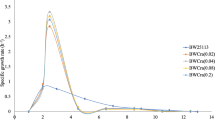
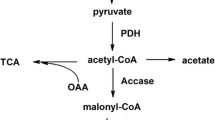
Abstract
Acetate production is one of the most striking differences between Escherichia coli K12 and BL21 strains. Transcription of acetate metabolism genes is regulated. Additionally, acetyl-CoA synthetase, which activates acetate to acetyl-CoA, is regulated by post-translational acetylation. The aim of this study was to understand the contribution of reversible protein lysine acetylation to the regulation of acetate metabolism in E. coli BL21. The phenotypic differences between both strains were especially important in the presence of acetate. The high expression of acetyl-CoA synthetase (acs) in glucose exponential phase in BL21 allows the simultaneous consumption of acetate and glucose. Lack of catabolite repression also affected its post-translational regulator, the protein acetyltransferase (patZ). The effect of the deletion of cobB (encoding a sirtuin-like protein deacetylase) and patZ genes depended on the genetic background. The deletion of cobB in both strains increased acetate production and decreased growth rate in acetate cultures. The deletion of patZ in BL21 suppressed acetate overflow in glucose medium and increased the growth rate in acetate cultures. Differences on acetate overflow between BL21 and K12 strains are caused by many overlapping factors. Two major contributing effects were identified: (1) the expression of acs during exponential growth is not repressed in the BL21 strain due to concomitant cAMP production and (2) the acetyl-CoA synthetase activity is more tightly regulated by protein acetylation in BL21 than in the K12. Altogether these differences contribute to the lower acetate overflow and the improved ability of E. coli BL21 to consume this metabolite in the presence of glucose.






Similar content being viewed by others
References
Aoshima M, Ishii M, Yamagishi A, Oshima T, Igarashi Y (2003) Metabolic characteristics of an isocitrate dehydrogenase defective derivative of Escherichia coli BL21(DE3). Biotechnol Bioeng 84:732–737. doi:10.1002/bit.10832
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10:411–21
Bergmeyer HU, Forster G, Bernt E (1974) Creatine kinase. Methods Enzym. Anal. pp 784–793
Bernal V, Castaño-Cerezo S, Gallego-Jara J, Ecija-Conesa A, de Diego T, Iborra JL, Cánovas M (2014) Regulation of bacterial physiology by lysine acetylation of proteins. N Biotechnol 31:586–95. doi:10.1016/j.nbt.2014.03.002
Castaño-Cerezo S, Bernal V, Blanco-Catalá J, Iborra JL, Cánovas M (2011) cAMP-CRP co-ordinates the expression of the protein acetylation pathway with central metabolism in Escherichia coli. Mol Microbiol 82:1110–1128
Castaño-Cerezo S, Bernal V, Post H, Fuhrer T, Cappadona S, Sánchez-Díaz NC, Sauer U, Heck AJR, Altelaar AFM, Cánovas M (2014) Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol Syst Biol 10(11):762
Crosby HA, Escalante-Semerena JC (2014) The acetylation motif in AMP-forming acyl-CoA synthetases contains residues critical for acetylation and recognition by the protein acetyltransferase Pat of Rhodopseudomonas palustris. J Bacteriol. doi:10.1128/JB.00004-14
Crosby HA, Rank KC, Rayment I, Escalante-Semerena JC (2012a) Structural insights into the substrate specificity of the Rhodopseudomonas palustris protein acetyltransferase RpPat: identification of a loop critical for recognition by RpPat. J Biol Chem 287:41392–404. doi:10.1074/jbc.M112.417360
Crosby HA, Pelletier DA, Hurst GB, Escalante-Semerena JC (2012b) System-wide studies of N-lysine acetylation in Rhodopseudomonas palustris reveals substrate specificity of protein acetyltransferases. J Biol Chem 287:15590–15601. doi:10.1074/jbc.M112.352104
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi:10.1073/pnas.120163297
De Mey M, De Maeseneire S, Soetaert W, Vandamme E (2007) Minimizing acetate formation in E. coli fermentations. J Ind Microbiol Biotechnol 34:689–700. doi:10.1007/s10295-007-0244-2
El-Mansi M, Cozzone AJ, Shiloach J, Eikmanns BJ (2006) Control of carbon flux through enzymes of central and intermediary metabolism during growth of Escherichia coli on acetate. Curr Opin Microbiol 9:173–179. doi:10.1016/j.mib.2006.02.002
Fischer E, Sauer U (2003) A novel metabolic cycle catalyzes glucose oxidation and anaplerosis in hungry Escherichia coli. J Biol Chem 278:46446–46451
Gardner JG, Escalante-Semerena JC (2008) Biochemical and mutational analyses of AcuA, the acetyltransferase enzyme that controls the activity of the acetyl coenzyme A synthetase (AcsA) in Bacillus subtilis. J Bacteriol 190:5132–5136
Gardner JG, Escalante-Semerena JC (2009) In Bacillus subtilis, the sirtuin protein deacetylase, encoded by the srtN gene (formerly yhdZ), and functions encoded by the acuABC genes control the activity of acetyl coenzyme A synthetase. J Bacteriol 191:1749–1755
Gardner JG, Grundy FJ, Henkin TM, Escalante-Semerena JC (2006) Control of acetyl-coenzyme a synthetase (AcsA) activity by acetylation/deacetylation without NAD+ involvement in Bacillus subtilis. J Bacteriol 188:5460–5468
Han M-J, Lee SY, Hong SH (2012) Comparative analysis of envelope proteomes in Escherichia coli B and K-12 strains. J Microbiol Biotechnol 22:470–8
Hayden JD, Brown LR, Gunawardena HP, Perkowski EF, Chen X, Braunstein M (2013) Reversible acetylation regulates acetate and propionate metabolism in Mycobacterium smegmatis. Microbiology 159:1986–99. doi:10.1099/mic. 0.068585-0
Jeong H, Barbe V, Lee CH, Vallenet D, Yu DS, Choi S-H, Couloux A, Lee S-W, Yoon SH, Cattolico L, Hur C-G, Park H-S, Ségurens B, Kim SC, Oh TK, Lenski RE, Studier FW, Daegelen P, Kim JF (2009) Genome sequences of Escherichia coli B strains REL606 and BL21(DE3). J Mol Biol 394:644–52. doi:10.1016/j.jmb.2009.09.052
Karp PD, Paley SM, Krummenacker M, Latendresse M, Dale JM, Lee TJ, Kaipa P, Gilham F, Spaulding A, Popescu L, Altman T, Paulsen I, Keseler IM, Caspi R (2010) Pathway Tools version 13.0: integrated software for pathway/genome informatics and systems biology. Brief Bioinform 11:40–79. doi:10.1093/bib/bbp043
Keseler IM, Mackie A, Peralta-Gil M, Santos-Zavaleta A, Gama-Castro S, Bonavides-Martínez C, Fulcher C, Huerta AM, Kothari A, Krummenacker M, Latendresse M, Muñiz-Rascado L, Ong Q, Paley S, Schröder I, Shearer AG, Subhraveti P, Travers M, Weerasinghe D, Weiss V, Collado-Vides J, Gunsalus RP, Paulsen I, Karp PD (2013) EcoCyc: fusing model organism databases with systems biology. Nucleic Acids Res 41:D605–12. doi:10.1093/nar/gks1027
Kleman GL, Strohl WR (1994) Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl Environ Microbiol 60:3952–3958
Lara AR, Caspeta L, Gosset G, Bolívar F, Ramírez OT (2008) Utility of an Escherichia coli strain engineered in the substrate uptake system for improved culture performance at high glucose and cell concentrations: an alternative to fed-batch cultures. Biotechnol Bioeng 99:893–901. doi:10.1002/bit.21664
Lima BP, Thanh Huyen TT, Bäsell K, Becher D, Antelmann H, Wolfe AJ (2012) Inhibition of acetyl phosphate-dependent transcription by an acetylatable lysine on RNA polymerase. J Biol Chem 287:32147–60. doi:10.1074/jbc.M112.365502
Lin H, Castro N, Bennett G, San K-Y (2006) Acetyl-CoA synthetase overexpression in Escherichia coli demonstrates more efficient acetate assimilation and lower acetate accumulation: a potential tool in metabolic engineering. Appl Microbiol Biotechnol 71:870–874. doi:10.1007/s00253-005-0230-4
Luli GW, Strohl WR (1990) Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol 56:1004–1011
Maharjan RP, Yu P-LL, Seeto S, Ferenci T, Prasad Maharjan R (2005) The role of isocitrate lyase and the glyoxylate cycle in Escherichia coli growing under glucose limitation. Res Microbiol 156:178–183. doi:10.1016/j.resmic.2004.09.004
Marisch K, Bayer K, Scharl T, Mairhofer J, Krempl PM, Hummel K, Razzazi-Fazeli E, Striedner G (2013) A comparative analysis of industrial Escherichia coli K-12 and B strains in high-glucose batch cultivations on process-, transcriptome- and proteome level. PLoS ONE 8:e70516. doi:10.1371/journal.pone.0070516
Meier S, Jensen PR, Duus JØ (2012) Direct observation of metabolic differences in living Escherichia coli strains K-12 and BL21. Chembiochem 13:308–10. doi:10.1002/cbic.201100654
Mischerikow N, Spedale G, Maarten Altelaar AF, Marc Timmers HT, Pim Pijnappel WWM, Heck AJR (2009) In-depth profiling of post-translational modifications on the related transcription factor complexes TFIID and SAGA. J Proteome Res 8:5020–5030
Nambi S, Basu N, Visweswariah S (2010) cAMP-regulated protein lysine acetylases in Mycobacteria. J Biol Chem 285:24313–24323. doi:10.1074/jbc.M110.118398
Negrete A, Majdalani N, Phue J-N, Shiloach J (2013) Reducing acetate excretion from E. coli K-12 by over-expressing the small RNA SgrS. N Biotechnol 30:269–73. doi:10.1016/j.nbt.2011.11.007
Nielsen J (2006) Microbial process kinetics. In Basic biotechnology. Ratledge, C.(ed.). Cambridge: Cambridge University Press, pp. 127-149
Peng L, Shimizu K (2003) Global metabolic regulation analysis for Escherichia coli K12 based on protein expression by 2-dimensional electrophoresis and enzyme activity measurement. Appl Microbiol Biotechnol 61:163–178
Phue J-N, Shiloach J (2004) Transcription levels of key metabolic genes are the cause for different glucose utilization pathways in E. coli B (BL21) and E. coli K (JM109). J Biotechnol 109:21–30. doi:10.1016/j.jbiotec.2003.10.038
Phue JN, Noronha SB, Hattacharyya R, Wolfe AJ, Shiloach J (2005) Glucose metabolism at high density growth of E. coli B and E. coli K: differences in metabolic pathways are responsible for efficient glucose utilization in E. coli B as determined by microarrays and northern blot analyses. Biotechnol Bioeng 90:805–820
Renilla S, Bernal V, Fuhrer T, Castaño-Cerezo S, Pastor JM, Iborra JL, Sauer U, Cánovas M (2012) Acetate scavenging activity in Escherichia coli: interplay of acetyl-CoA synthetase and the PEP-glyoxylate cycle in chemostat cultures. Appl Microbiol Biotechnol 95:2109–2124. doi:10.1007/s00253-011-3536-4
Shiloach J, Kaufman J, Guillard AS, Fass R (1996) Effect of glucose supply strategy on acetate accumulation, growth, and recombinant protein production by Escherichia coli BL21 (lambda DE3) and Escherichia coli JM109. Biotechnol Bioeng 49:421–428
Son Y-J, Phue J-N, Trinh LB, Lee SJ, Shiloach J (2011) The role of Cra in regulating acetate excretion and osmotic tolerance in E. coli K-12 and E. coli B at high density growth. Microb Cell Fact 10:52. doi:10.1186/1475-2859-10-52
Starai VJ, Escalante-Semerena JC (2004) Acetyl-coenzyme A synthetase (AMP forming). Cell Mol Life Sci 61:2020–2030
Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC (2002) Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298(80):2390–2392. doi:10.1126/science.1077650
Studier FW, Daegelen P, Lenski RE, Maslov S, Kim JF (2009) Understanding the differences between genome sequences of Escherichia coli B strains REL606 and BL21(DE3) and comparison of the E. coli B and K-12 genomes. J Mol Biol 394:653–80. doi:10.1016/j.jmb.2009.09.021
Thao S, Escalante-semerena JC (2011) Biochemical and thermodynamic analyses of Salmonella enterica Pat, a multidomain, multimeric N(ε)-lysine acetyltransferase involved in carbon and energy metabolism. MBio 2:1–8. doi:10.1128/mBio.00216-11.Editor
Valgepea K, Adamberg K, Nahku R, Lahtvee P-J, Arike L, Vilu R (2010) Systems biology approach reveals that overflow metabolism of acetate in Escherichia coli is triggered by carbon catabolite repression of acetyl-CoA synthetase. BMC Syst Biol 4:166
Van de Walle M, Shiloach J (1998) Proposed mechanism of acetate accumulation in two recombinant Escherichia coli strains during high density fermentation. Biotechnol Bioeng 57:71–78
Waegeman H, Beauprez J, Moens H, Maertens J, De Mey M, Foulquie-Moreno M, Heijnen J, Charlier D, Soetaert W (2011) Effect of iclR and arcA knockouts on biomass formation and metabolic fluxes in Escherichia coli K-12 and its implications on understanding the metabolism of Escherichia coli BL21 (DE3). BMC Microbiol 11:70
Waegeman H, Maertens J, Beauprez J, De Mey M, Soetaert W (2012) Effect of iclR and arcA deletions on physiology and metabolic fluxes in Escherichia coli BL21 (DE3). Biotechnol Lett 34:329–37. doi:10.1007/s10529-011-0774-6
Walsh K, Koshland DE (1984) Determination of flux through the branch point of two metabolic cycles. The tricarboxylic acid cycle and the glyoxylate shunt. J Biol Chem 259:9646–9654
Weinert BTTT, Iesmantavicius V, Wagner SAAA, Schölz C, Gummesson B, Beli P, Nyström T, Choudhary C, Scho C, Nystro T (2013) Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol Cell 51:1–8. doi:10.1016/j.molcel.2013.06.003
Wolfe AJ (2005) The acetate switch. Microbiol Mol Biol Rev 69:12–50
Zhao K, Chai X, Marmorstein R (2004) Structure and substrate binding properties of CobB, a Sir2 homolog protein deacetylase from Escherichia coli. J Mol Biol 337:731–741
Acknowledgments
We wish to thank José María Pastor (Dept. of Biochemistry and Molecular Biology B and Immunology) for helpful discussions, Marta Abrisqueta, Elena Martín-Orozco and David Cerezo (Dept. of Biochemistry and Molecular Biology B and Immunology, University of Murcia) for their help with western blotting and Professor Kerry Smith (Clemson University, South Carolina) for his assistance with the acetate kinase assay. S. Castaño-Cerezo is a recipient of a Ph.D. fellowship from Fundación Séneca (CARM, Murcia). V. Bernal acknowledges a post-doctoral contract from Universidad de Murcia (Programa Propio). This work has been partly funded by MICINN BIO2011-29233-C02-01 and Fundación Séneca-CARM 08660/PI/08 projects.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 60 kb)
Rights and permissions
About this article
Cite this article
Castaño-Cerezo, S., Bernal, V., Röhrig, T. et al. Regulation of acetate metabolism in Escherichia coli BL21 by protein Nε-lysine acetylation. Appl Microbiol Biotechnol 99, 3533–3545 (2015). https://doi.org/10.1007/s00253-014-6280-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6280-8