Abstract
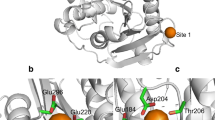
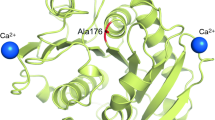
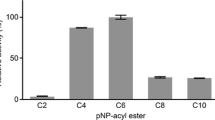
A cutinase-like enzyme from Saccharomonospora viridis AHK190, Cut190, hydrolyzes the inner block of polyethylene terephthalate (PET); this enzyme is a member of the lipase family, which contains an α/β hydrolase fold and a Ser-His-Asp catalytic triad. The thermostability and activity of Cut190 are enhanced by high concentrations of calcium ions, which is essential for the efficient enzymatic hydrolysis of amorphous PET. Although Ca2+-induced thermostabilization and activation of enzymes have been well explored in α-amylases, the mechanism for PET-degrading cutinase-like enzymes remains poorly understood. We focused on the mechanisms by which Ca2+ enhances these properties, and we determined the crystal structures of a Cut190 S226P mutant (Cut190S226P) in the Ca2+-bound and free states at 1.75 and 1.45 Å resolution, respectively. Based on the crystallographic data, a Ca2+ ion was coordinated by four residues within loop regions (the Ca2+ site) and two water molecules in a tetragonal bipyramidal array. Furthermore, the binding of Ca2+ to Cut190S226P induced large conformational changes in three loops, which were accompanied by the formation of additional interactions. The binding of Ca2+ not only stabilized a region that is flexible in the Ca2+-free state but also modified the substrate-binding groove by stabilizing an open conformation that allows the substrate to bind easily. Thus, our study explains the structural basis of Ca2+-enhanced thermostability and activity in PET-degrading cutinase-like enzyme for the first time and found that the inactive state of Cut190S226P is activated by a conformational change in the active-site sealing residue, F106.






Similar content being viewed by others
References
Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66:213–221
Amada K, Kwon HJ, Haruki M, Morikawa M, Kanaya S (2001) Ca2+-induced folding of a family 1.3 lipase with repetitive Ca2+ binding motifs at the C-terminus. FEBS Lett 509:17–21
Arockiasamy A, Aggarwal A, Savva CG, Holzenburg A, Sacchettini JC (2011) Crystal structure of calcium dodecin (Rv0379), from Mycobacterium tuberculosis with a unique calcium-binding site. Protein Sci 20:827–833
Bornscheuer UT (2002) Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol Rev 26:73–81
Cowtan K (2008) Fitting molecular fragments into electron density. Acta Crystallogr D Biol Crystallogr 64:83–89
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos
Emsley P, Lohkamp B, Scott W, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66:486–501
Herrero Acero E, Ribitsch D, Dellacher A, Zitzenbacher S, Marold A, Steinkellner G, Gruber K, Schwab H, Guebitz GM (2013) Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol Bioeng 110:2581–2590
Kabsch W (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr 26:795–800
Kawai F, Oda M, Tamashiro T, Waku T, Tanaka N, Yamamoto M, Mizushima H, Miyakawa T, Tanokura M (2014) A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl Microbiol Biotechnol 95:419–430
Kim MH, Kim HK, Lee JK, Park SY, Oh TK (2000) Thermostable lipase of Bacillus stearothermophilus: high-level production, purification, and calcium-dependent thermostability. Biosci Biotechnol Biochem 64:280–286
Kitadokoro K, Thumarat U, Nakamura R, Nishimura K, Karatani H, Suzuki H, Kawai F (2012) Crystal structure of cutinase Est119 from Thermobifida alba AHK119 that can degrade modified polyethylene terephthalate at 1.76 Å resolution. Polym Degrad Stab 97:771–775
Lovell SC, Davis IW, Arendall WB III, de Bakker PIW, Word JM, Prisant MG, Richardson JS, Richardson DC (2003) Structure validation by Cα geometry: φ, ψ and Cβ deviation. Proteins 50:437–450
Machius M, Wiegand G, Huber R (1995) Crystal structure of calcium-depleted Bacillus licheniformis alpha-amylase at 2.2 Å resolution. J Mol Biol 246:545–559
Machius M, Declerck N, Huber R, Wiegand G (1998) Activation of Bacillus licheniformis alpha-amylase through a disorder → order transition of the substrate-binding site mediated by a calcium-sodium-calcium metal triad. Structure 6:281–292
Morris RJ, Perrakis A, Lamzin VS (2002) ARP/wARP’s model-building algorithms. I. The main chain. Acta Crystallogr D Biol Crystallogr 58:968–975
Müller RJ, Schrader H, Profe J, Dresler K, Deckwer WD (2005) Enzymatic degradation of poly(ethylene terephthalate): rapid hydrolysis using a hydrolase from Thermobifida fusca. Macromol Rapid Commun 26:1400–1405
Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255
Niesen FH, Berglund H, Vadadi M (2007) The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2:2212–2221
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326
Pinsirodom P, Parkin KL (2001) Current protocols in food analytical chemistry. John Wiley & Sons, New York
Pio TF, Macedo GA (2009) Cutinases: properties and industrial applications. Adv Appl Microbiol 66:77–95
Ribitsch D, Yebra AO, Zitzenbacher S, Wu J, Nowitsch S, Steinkellner G, Greimel K, Doliska A, Oberdorfer G, Gruber CC, Gruber K, Schwab H, Stana-Kleinschek K, Acero EH, Guebitz GM (2013) Fusion of binding domains to Thermobifida cellulosilytica cutinase to tune sorption characteristics and enhancing PET hydrolysis. Biomacromolecules 14:1769–1776
Ronkvist Å, Xie W, Lu W, Gross RA (2009) Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 42:5128–5138
Roth C, Wei R, Oeser T, Then J, Föllner C, Zimmermann W, Sträter N (2014) Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl Microbiol Biotechnol, in press
Simons JFA, Van Kampen MD, Ubarretxena-Belandia I, Cox RC, Alves dos Santos CM, Egmond MR, Verheij HM (1999) Identification of a calcium binding site in Staphylococcus hyicus lipase: generation of calcium-independent variants. Biochemistry 38:2–10
Sulaiman S, You D-J, Kanaya E, Koga Y, Kanaya S (2014) Crystal structure and thermodynamic and kinetic stability of metagenome-derived LC-cutinase. Biogeosciences 53:1858–1869
Thumarat U, Nakamura R, Kawabata T, Suzuki H, Kawai F (2012) Biochemical and genetic analysis of a cutinase-type polyesterase from a thermophilic Thermobifida alba AHK119. Appl Microbiol Biotechnol 95:419–430
Vagin A, Teplyakov A (1997) MOLREP: an automated program for molecular replacement. J Appl Crystallogr 30:1022–1025
Vertommen MA, Nierstrasz VA, Veer Mv, Warmoeskerken MM (2005) Enzymatic surface modification of poly(ethylene terephthalate). J Biotechnol 120:376–386
Wei R, Oeser T, Then J, Kühn N, Barth M, Schmidt J, Zimmermann W (2014) Functional characterization and structural modeling of synthetic polyester-degrading hydrolases from Thermomonospora curvata. AMB Express 4:44
Yadav JK (2012) A differential behavior of α-amylase, in terms of catalytic activity and thermal stability, in response to higher concentration CaCl2. Int J Biol Macromol 51:146–2152
Zhang Y, Wang L, Chen J, Wu J (2013) Enhanced activity toward PET by site-directed mutagenesis of Thermobifida fusca cutinase-CBM fusion protein. Carbohydr Polym 97:124–129
Zimmermann W, Billig S (2011) Enzymes for the biofunctionalization of poly(ethylene terephthalate). Adv Biochem Engin/Biotechnol 125:97–120
Acknowledgments
This work was supported by the Platform for Drug Design, Discovery and Development of the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT). We would like to thank the scientists and staff at the Photon Factory. The synchrotron radiation experiments were conducted at the beamlines AR-NW12 and BL-17A at the Photon Factory, Tsukuba, Japan (Proposal No. 2013G658).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyakawa, T., Mizushima, H., Ohtsuka, J. et al. Structural basis for the Ca2+-enhanced thermostability and activity of PET-degrading cutinase-like enzyme from Saccharomonospora viridis AHK190. Appl Microbiol Biotechnol 99, 4297–4307 (2015). https://doi.org/10.1007/s00253-014-6272-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6272-8