Abstract
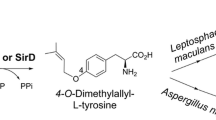
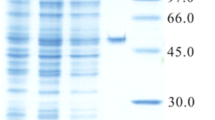
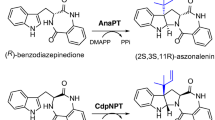
During our search for novel prenyltransferases, a putative gene ATEG_04218 from Aspergillus terreus raised our attention and was therefore amplified from strain DSM 1958 and expressed in Escherichia coli. Biochemical investigations with the purified recombinant protein and different aromatic substrates in the presence of dimethylallyl diphosphate revealed the acceptance of all the tested tryptophan-containing cyclic dipeptides. Structure elucidation of the main enzyme products by NMR and MS analyses confirmed the attachment of the prenyl moiety to C-7 of the indole ring, proving the identification of a cyclic dipeptide C7-prenyltransferase (CdpC7PT). For some substrates, reversely C3- or N1-prenylated derivatives were identified as minor products. In comparison to the known tryptophan-containing cyclic dipeptide C7-prenyltransferase CTrpPT from Aspergillus oryzae, CdpC7PT showed a much higher substrate flexibility. It also accepted cyclo-l-Tyr-l-Tyr as substrate and catalyzed an O-prenylation at the tyrosyl residue, providing the first example from the dimethylallyltryptophan synthase (DMATS) superfamily with an O-prenyltransferase activity towards dipeptides. Furthermore, products with both C7-prenyl at tryptophanyl and O-prenyl at tyrosyl residue were detected in the reaction mixture of cyclo-l-Trp-l-Tyr. Determination of the kinetic parameters proved that (S)-benzodiazepinedione consisting of a tryptophanyl and an anthranilyl moiety was accepted as the best substrate with a K M value of 204.1 μM and a turnover number of 0.125 s−1. Cyclo-l-Tyr-l-Tyr was accepted with a K M value of 1,411.3 μM and a turnover number of 0.012 s−1.




Similar content being viewed by others
References
Ames BD, Walsh CT (2010) Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochemistry 49:3351–3365
Arai K, Sato S, Shimizu S, Nitta K, Yamamoto Y (1981) Metabolic products of Aspergillus terreus. VII. Astechrome: an iron-containing metabolite of the strain IFO 6123. Chem Pharm Bull 29:1510–1517
Birch AJ, Blance GE, David S, Smith H (1961) Studies in relation to biosynthesis. XXIV. Some remarks on the structure of echinuline. J Chem Soc 3128–3131
Bonitz T, Alva V, Saleh O, Lupas AN, Heide L (2011) Evolutionary relationships of microbial aromatic prenyltransferases. PLoS One 6:e27336
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Guo CJ, Knox BP, Sanchez JF, Chiang YM, Bruno KS, Wang CCC (2013) Application of an efficient gene targeting system linking secondary metabolites to their biosynthetic genes in Aspergillus terreus. Org Lett 3562–3565
Heide L (2009) Prenyl transfer to aromatic substrates: genetics and enzymology. Curr Opin Chem Biol 13:171–179
Jain HD, Zhang C, Zhou S, Zhou H, Ma J, Liu X, Liao X, Deveau AM, Dieckhaus CM, Johnson MA, Smith KS, Macdonald TL, Kakeya H, Osada H, Cook JM (2008) Synthesis and structure-activity relationship studies on tryprostatin A, a potent inhibitor of breast cancer resistance protein. Bioorg Med Chem 16:4626–4651
Jeedigunta S, Krenisky JM, Kerr RG (2000) Diketopiperazines as advanced intermediates in the biosynthesis of ecteinascidins. Tetrahedron 56:3303–3307
Kremer A, Li S-M (2010) A tyrosine O-prenyltransferase catalyses the first pathway-specific step in the biosynthesis of sirodesmin PL. Microbiology 156:278–286
Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409–3416
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Lu YP, Liu HG, Liang PH (2009) Different reaction mechanisms for cis- and trans-prenyltransferases. Biochem Biophys Res Commun 379:351–355
Mundt K, Li S-M (2013) CdpC2PT, a reverse prenyltransferase from Neosartorya fischeri with distinct substrate preference from known C2-prenyltransferases. Microbiology 159:2169–2179
Pockrandt D, Sack C, Kosiol T, Li S-M (2014) A promiscuous prenyltransferase from Aspergillus oryzae catalyses C-prenylations of hydroxynaphthalenes in the presence of different prenyl donors. Appl Microbiol Biotechnol 98:4987–4994
Rudolf JD, Poulter CD (2013) Tyrosine O-prenyltransferase SirD catalyzes S-, C-, and N-prenylations on tyrosine and tryptophan derivatives. ACS Chem Biol 8:2707–2714
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99
Steffan N, Li S-M (2009) Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch Microbiol 191:461–466
Steffan N, Grundmann A, Yin W-B, Kremer A, Li S-M (2009) Indole prenyltransferases from fungi: a new enzyme group with high potential for the production of prenylated indole derivatives. Curr Med Chem 16:218–231
Tarcz S, Ludwig L, Li S-M (2014) AstPT catalyses both reverse N1- and regular C2-prenylation of a methylated bisindolyl benzoquinone. Chembiochem 15:108–116
Tsai HF, Wang H, Gebler JC, Poulter CD, Schardl CL (1995) The Claviceps purpurea gene encoding dimethylallyltryptophan synthase, the committed step for ergot alkaloid biosynthesis. Biochem Biophys Res Commun 216:119–125
Unsöld IA, Li S-M (2005) Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499–1505
Wang Y, Gloer JB, Scott JA, Malloch D (1995) Terezines A-D: new amino acid-derived bioactive metabolites from the coprophilous fungus Sporormiella teretispora. J Nat Prod 58:93–99
Wang WL, Lu ZY, Tao HW, Zhu TJ, Fang YC, Gu QQ, Zhu WM (2007) Isoechinulin-type alkaloids, variecolorins A-L, from halotolerant Aspergillus variecolor. J Nat Prod 70:1558–1564
Williams RM, Stocking EM, Sanz-Cervera JF (2000) Biosynthesis of prenylated alkaloids derived from tryptophan. Top Curr Chem 209:97–173
Winkelblech J, Li S-M (2014) Biochemical investigations of two 6-DMATS enzymes from Streptomyces revealing novel features of L-tryptophan prenyltransferases. Chembiochem 15:1030–1039
Wollinsky B, Ludwig L, Hamacher A, Yu X, Kassack MU, Li S-M (2012a) Prenylation at the indole ring leads to a significant increase of cytotoxicity of tryptophan-containing cyclic dipeptides. Bioorg Med Chem Lett 22:3866–3869
Wollinsky B, Ludwig L, Xie X, Li S-M (2012b) Breaking the regioselectivity of indole prenyltransferases: identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270
Woodside AB, Huang Z, Poulter CD (1988) Trisammonium geranyl diphosphate. Org Synth 66:211–215
Yazaki K, Sasaki K, Tsurumaru Y (2009) Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 70:1739–1745
Yin W-B, Ruan H-L, Westrich L, Grundmann A, Li S-M (2007) CdpNPT, an N-prenyltransferase from Aspergillus fumigatus: overproduction, purification and biochemical characterisation. Chembiochem 8:1154–1161
Yin W-B, Grundmann A, Cheng J, Li S-M (2009) Acetylaszonalenin biosynthesis in Neosartorya fischeri: identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem 284:100–109
Yin W-B, Xie X-L, Matuschek M, Li S-M (2010a) Reconstruction of pyrrolo[2,3-b]indoles carrying an α-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org Biomol Chem 8:1133–1141
Yin W-B, Yu X, Xie X-L, Li S-M (2010b) Preparation of pyrrolo[2,3-b]indoles carrying a ß-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org Biomol Chem 8:2430–2438
Yin S, Yu X, Wang Q, Liu XQ, Li S-M (2013a) Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 97:1649–1660
Yin W-B, Baccile JA, Bok JW, Chen Y, Keller NP, Schroeder FC (2013b) A nonribosomal peptide synthetase-derived iron(III) complex from the pathogenic fungus Aspergillus fumigatus. J Am Chem Soc 135:2064–2067
Yu X, Li S-M (2012) Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol 516:259–278
Yu X, Liu Y, Xie X, Zheng X-D, Li S-M (2012) Biochemical characterization of indole prenyltransferases: filling the last gap of prenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J Biol Chem 287:1371–1380
Yu X, Zocher G, Xie X, Liebhold M, Schütz S, Stehle T, Li S-M (2013) Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem Biol 20:1492–1501
Zou H-X, Xie X-L, Linne U, Zheng X-D, Li S-M (2010) Simultaneous C7- and N1-prenylation of cyclo-L-Trp-L-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org Biomol Chem 8:3037–3044
Zou H-X, Xie X, Zheng X-D, Li S-M (2011) The tyrosine O-prenyltransferase SirD catalyzes O-, N-, and C-prenylations. Appl Microbiol Biotechnol 89:1443–1451
Acknowledgments
This project was financially supported by a grant from the Deutsche Forschungsgemeinschaft (Li844/4-1 to S.-M. Li). We thank L. Ludwig for synthesis of DMAPP. We also thank analytic department of the faculty of Pharmacy for taking mass and NMR spectra, respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 11725 kb)
Rights and permissions
About this article
Cite this article
Wunsch, C., Zou, HX., Linne, U. et al. C7-prenylation of tryptophanyl and O-prenylation of tyrosyl residues in dipeptides by an Aspergillus terreus prenyltransferase. Appl Microbiol Biotechnol 99, 1719–1730 (2015). https://doi.org/10.1007/s00253-014-5999-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5999-6