Abstract
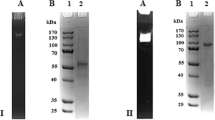
Data on glucose dehydrogenases (GDHs) are scarce and availability of these enzymes for application purposes is limited. This paper describes a new GDH from the fungus Pycnoporus cinnabarinus CIRM BRFM 137 that is the first reported GDH from a white-rot fungus belonging to the Basidiomycota. The enzyme was recombinantly produced in Aspergillus niger, a well-known fungal host producing an array of homologous or heterologous enzymes for industrial applications. The full-length gene that encodes GDH from P. cinnabarinus (PcGDH) consists of 2,425 bp and codes for a deduced protein of 620 amino acids with a calculated molecular mass of 62.5 kDa. The corresponding complementary DNA was cloned and placed under the control of the strong and constitutive glyceraldehyde-3-phosphate dehydrogenase promoter. The signal peptide of the glucoamylase prepro sequence of A. niger was used to target PcGDH secretion into the culture medium, achieving a yield of 640 mg L−1, which is tenfold higher than any other reported value. The recombinant PcGDH was purified twofold to homogeneity in a one-step procedure with a 41 % recovery using a Ni Sepharose column. The identity of the recombinant protein was further confirmed by immunodetection using western blot analysis and N-terminal sequencing. The molecular mass of the native PcGDH was 130 kDa, suggesting a homodimeric form. Optimal pH and temperature were found to be similar (5.5 and 60 °C, respectively) to those determined for the previously characterized GDH, i.e., from Glomerella cingulata. However PcGDH exhibits a lower catalytic efficiency of 67 M−1 s−1 toward glucose. This substrate is by far the preferred substrate, which constitutes an advantage over other sugar oxidases in the case of blood glucose monitoring. The substrate-binding domain of PcGDH turns out to be conserved as compared to other glucose-methanol-choline (GMCs) oxidoreductases. In addition, the ability of PcGDH to reduce oxidized quinones or radical intermediates was clearly demonstrated, which raises prospects for applying this enzyme to detoxify toxic compounds formed during the degradation of lignin.






Similar content being viewed by others

References
Aiba H, Tsugura-shi J (2007) Novel glucose dehydrogenase. US Patent 2007/0105174
Armstrong JM (1964) The molar extinction coefficient of 2,6-dichlorophenol indophenol. Biochim Biophys Acta 86:194–197
Bai L, Wen D, Yin J, Deng L, Zhu C, Dong S (2012) Carbon nanotubes-ionic liquid nanocomposites sensing platform for NADH oxidation and oxygen, glucose detection in blood. Talanta 91:110–115
Bak TG (1967) Studies on glucose dehydrogenase of Aspergillus oryzae. Purification and physical and chemical properties. Biochim Biophys Acta 139:277–293
Bak TG, Sato R (1967) Studies on the glucose dehydrogenase of Aspergillus oryzae. Induction of its synthesis by ρ-benzoquinone and hydroquinone. Biochim Biophys Acta 139:265–276
Bey M, Berrin JG, Poidevin L, Sigoillot JC (2011) Heterologous expression of Pycnoporus cinnabarinus cellobiose dehydrogenase in Pichia pastoris and involvement in saccharification processes. Microb Cell Factories 10:113–127
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238
Clark LC Jr, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102:29–45
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Estrada Alvarado I, Lomascolo A, Navarro D, Delattre M, Asther M, Lesage-Meessen L (2001) Evidence of a new biotransformation pathway of p-coumaric acid into p-hydroxybenzaldehyde in Pycnoporus cinnabarinus. Appl Microbiol Biotechnol 57:725–730
Ferri S, Kojima K, Sode K (2011) Review of glucose oxidases and glucose dehydrogenases: a bird’s eye view of glucose sensing enzymes. J Diabetes Sci Technol 5:1068–1076
Forney LJ, Reddy CA, Pankratz HS (1982) Ultrastructural localization of hydrogen peroxide production in ligninolytic Phanerochaete chrysosporium cells. Appl Environ Microbiol 44:732–736
Gordon CL, Khalaj V, Ram AFJ, Archer DB, Brookman JL, Trinci APJ, Jeenes DJ, Doonan JH, Wells B, Punt PJ, van den Hondel CAMJJ, Robson GD (2000) Glucoamylase: fluorescence protein fusions to monitor protein secretion in Aspergillus niger. Microbiology 146:415–426
Harrison DC (1931) Glucose dehydrogenase: a new oxidising enzyme from animal tissues. Biochem J 4:1016–1027
Hecht HJ, Kalisz HM, Hendle J, Schmid RD, Schomburg D (1993) Crystal structure of glucose oxidase from Aspergillus niger refined at 2.3 A resolution. J Mol Biol 1:153–172
Kelley RL, Reddy CA (1986) Purification and characterization of glucose oxidase from ligninolytic cultures of Phanerochaete chrysosporium. J Bacteriol 166:269–274
Kiess M, Hecht HJ, Kalisz HM (1998) Glucose oxidase from Penicillium amagasakiense. Primary structure and comparison with other glucose-methanol-choline (GMC) oxidoreductases. Eur J Biochem 252:90–99
Kujawa M, Volc J, Halada P, Sedmera P, Divne C, Sygmund C, Leitner C, Peterbauer C, Haltrich D (2007) Properties of pyranose dehydrogenase purified from the litter-degrading fungus Agaricus xanthoderma. FEBS J 274:879–894
Lesage-Meessen L, Delattre M, Haon M, Thibault JF, Ceccaldi BC, Brunerie P, Asther M (1996) A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. J Biotechnol 50:107–113
Leskovac V, Trivić S, Wohlfahrt G, Kandrac J, Pericin D (2005) Glucose oxidase from Aspergillus niger: the mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int J Biochem Cell Biol 37:731–750
Levasseur A, Drula E, Lombard V, Coutinho PM, Henrissat B (2013) Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol Biofuels 6:41–54
Levasseur A, Lomascolo A, Chabrol O, Ruiz-Dueñas FJ, Boukhris-Uzan E, Piumi F, Kües U, Ram AFJ, Murat C, Haon M, Benoit I, Arfi Y, Chevret D, Drula E, Kwon MJ, Gouret P, Lesage-Meessen L, Lombard V, Mariette J, Noirot C, Park J, Patyshakuliyeva A, Sigoillot JCS, Wiebenga A, Wösten HAB, Martin F, Coutinho PM, de Vries RP, Martínez AT, Klopp C, Pontarotti P, Henrissat B, Record E (2014) The genome of the white-rot fungus Pycnoporus cinnabarinus: a basidiomycete model with a versatile arsenal for lignocellulosic biomass breakdown. BMC Genomics In press
Lomascolo A, Stentelaire C, Asther M, Lesage-Meessen L (1999) Basidiomycetes as new biotechnological tools to generate natural aromatic flavours for the food industry. Trends Biotechnol 17:282–289
Lomascolo A, Record E, Herpoël-Gimbert I, Delattre M, Robert JL, Georis J, Dauvrin T, Sigoillot JC, Asther M (2003) Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J Appl Microbiol 94:618–624
Lomascolo A, Uzan-Boukhris E, Herpoël-Gimbert I, Sigoillot JC, Lesage-Meessen L (2011) Peculiarities of Pycnoporus species for applications in biotechnology. Appl Microbiol Biotechnol 92:1129–1149
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Madzak C, Otterbein L, Chamkha M, Moukha S, Asther M, Gaillardin C, Beckerich JM (2005) Heterologous production of a laccase from the basidiomycete Pycnoporus cinnabarinus in the dimorphic yeast Yarrowia lipolytica. FEMS Yeast Res 5:635–646
Mekmouche Y, Zhou S, Cusano AM, Record E, Lomascolo A, Robert V, Simaan AJ, Rousselot-Pailley P, Ullah S, Chaspoul F, Tron T (2014) Gram-scale production of a basidiomycetous laccase in Aspergillus niger. J Biosci Bioeng 117:25–27
Milutinovic M, Sallard S, Manojlovic D, Mano N, Sojic N (2011) Glucose sensing by electrogenerated chemiluminescence of glucose-dehydrogenase produced NADH on electrodeposited redox hydrogel. Bioelectrochemistry 82:63–68
Mori K, Nakajima M, Kojima K, Murakami K, Ferri S, Sode K (2011) Screening of Aspergillus-derived FAD-glucose dehydrogenases from fungal genome database. Biotechnol Lett 33:2255–2263
Müller HM (1977) Gluconic acid forming enzymes in Aspergillus niger. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg 132:14–24
Ogura Y (1951) Studies on the glucose dehydrogenase of Aspergillus oryzae. J Biochem 38:75–84
Ogura Y, Nagahisa M (1937) Untersuchungen über die Atmung und die Dehydrasesysteme von Aspergillus oryzae. Bot Mag Tokyo 51:597–612
Otterbein L, Record E, Longhi S, Asther M, Moukha S (2000) Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur J Biochem 267:1619–1625
Poidevin L, Levasseur A, Paës G, Navarro D, Heiss-Blanquet S, Asther M, Record E (2009) Heterologous production of the Piromyces equi cinnamoyl esterase in Trichoderma reesei for biotechnological applications. Lett Appl Microbiol 49:673–678
Punt PJ, van den Hondel CA (1992) Transformation of filamentous fungi based on hygromycin B and phleomycine resistance markers. Methods Enzymol 216:447–457
Record E, Punt PJ, Chamkha M, Labat M, van Den Hondel CA, Asther M (2002) Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur J Biochem 269:602–609
Saleh FS, Mao L, Ohsaka T (2012) A promising dehydrogenase-based bioanode for a glucose biosensor and glucose/O2 biofuel cell. Analyst 137:2233–2238
Shin KS, Youn HD, Han YH, Kang SO, Hah YC (1993) Purification and characterisation of D-glucose oxidase from white-rot fungus Pleurotus ostreatus. Eur J Biochem 215:747–752
Sigoillot C, Record E, Belle V, Robert JL, Levasseur A, Punt PJ, van den Hondel CA, Fournel A, Sigoillot JC, Asther M (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Swoboda BE, Massey V (1965) Purification and properties of the glucose oxidase from Aspergillus niger. J Biol Chem 240:2209–2215
Sygmund C, Klausberger M, Felice AK, Ludwig R (2011a) Reduction of quinones and phenoxy radicals by extracellular glucose dehydrogenase from Glomerella cingulata suggests a role in plant pathogenicity. Microbiology 157:3203–3212
Sygmund C, Staudigl P, Klausberger M, Pinotsis N, Djinović-Carugo K, Gorton L, Haltrich D, Ludwig R (2011b) Heterologous overexpression of Glomerella cingulata FAD-dependent glucose dehydrogenase in Escherichia coli and Pichia pastoris. Microb Cell Factories 10:106–114
Takegawa K, Fujiwara K, Iwahara S, Yamamoto K, Tochikura T (1989) Effect of deglycosylation of N-linked sugar chains on glucose oxidase from Aspergillus niger. Biochem Cell Biol 67:460–464
Tan TC, Spadiut O, Wongnate T, Sucharitakul J, Krondorfer I, Sygmund C, Haltrich D, Chaiyen P, Peterbauer CK, Divne C (2013) The 1.6 Å crystal structure of pyranose dehydrogenase from Agaricus meleagris rationalizes substrate specificity and reveals a flavin intermediate. PLoS ONE 8:1–14
Tsujimura S, Kojima S, Kano K, Ikeda T, Sato M, Sanada H, Omura H (2006) Novel FAD-dependent glucose dehydrogenase for a dioxygen-insensitive glucose biosensor. Biosci Biotechnol Biochem 70:654–659
Turbe-Doan A, Arfi Y, Record E, Estrada-Alvarado I, Levasseur (2013) A Heterologous production of cellobiose dehydrogenases from the basidiomycete Coprinopsis cinerea and the ascomycete Podospora anserina and their effect on saccharification of wheat straw. Appl Microbiol Biotechnol 97:4873–4885
van Hartingsveldt W, Mattern IE, van Zeijl CM, Pouwells PH, van den Hondel CA (1987) Development of a homologous transformation system for Aspergillus niger based on the pyrG gene. Mol Gen Genet 206:71–75
Wang JY, Nien PC, Chen CH, Chen LC, Ho KC (2012) A glucose bio-battery prototype based on a GDH/poly(methylene blue) bioanode and a graphite cathode with an iodide/tri-iodide redox couple. Bioresour Technol 116:502–506
Wohlfahrt G, Witt S, Hendle J, Schomburg D, Kalisz HM, Hecht HJ (1999) 1.8 and 1.9 A resolution structures of the Penicillium amagasakiense and Aspergillus niger glucose oxidases as a basis for modelling substrate complexes. Acta Crystallogr D Biol Crystallogr 55:969–977
Wong CM, Wong KH, Chen XD (2008) Glucose oxidase: natural occurrence, function, properties and industrial applications. Appl Microbiol Biotechnol 78:927–938
Yang Y, Wei B, Zhao Y, Wang J (2013) Construction of an integrated enzyme system consisting azoreductase and glucose 1-dehydrogenase for dye removal. Bioresour Technol 130:517–521
Zafar MN, Wang X, Sygmund C, Ludwig R, Leech D, Gorton L (2012) Electron-transfer studies with a new flavin adenine dinucleotide dependent glucose dehydrogenase and osmium polymers of different redox potentials. Anal Chem 84:334–341
Zhang L, Zhou M, Wen D, Bai L, Lou B, Dong S (2012) Small-size biofuel cell on paper. Biosens Bioelectron 35:155–159
Acknowledgments
This work was partly funded by the European Commission under the INDOX project (KBBE-2013-7-613549). The authors thank Régine Lebrun, from the proteomics platform of the Institut de Microbiologie de la Méditerranée, CNRS-AMU, Marseille, France, for protein identification by mass spectrometry, and Christophe Boyer and Marie-Pierre Forquin-Gomez for the graphics.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 136 kb)
Rights and permissions
About this article
Cite this article
Piumi, F., Levasseur, A., Navarro, D. et al. A novel glucose dehydrogenase from the white-rot fungus Pycnoporus cinnabarinus: production in Aspergillus niger and physicochemical characterization of the recombinant enzyme. Appl Microbiol Biotechnol 98, 10105–10118 (2014). https://doi.org/10.1007/s00253-014-5891-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5891-4