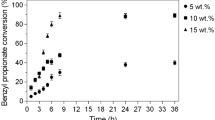
Abstract
Taking advantage of the catalytic promiscuity of l-carbamoylase from Geobacillus stearothermophilus CECT43 (BsLcar) and N-succinyl-amino acid racemase from Geobacillus kaustophilus CECT4264 (GkNSAAR), we have evaluated the production of different optically pure l-α-amino acids starting from different racemic N-formyl- and N-carbamoyl-amino acids using a dynamic kinetic resolution approach. The enzymes were immobilized on two different solid supports, resulting in improved stability of the enzymes in terms of thermostability and storage when compared to the enzymes in solution. The bienzymatic system retained up to 80 % conversion efficiency after 20 weeks at 4 °C and up to 90 % after 1 week at 45 °C. The immobilization process also resulted in a great enhancement of the activity of BsLcar toward N-formyl-tryptophan, showing for the first time that substrate specificity of l-carbamoylases can be influenced by this approach. The system was effective for the biosynthesis of natural and unnatural l-amino acids (enantiomeric excess (e.e.) >99.5 %), such as l-methionine, l-alanine, l-tryptophan, l-homophenylalanine, l-aminobutyric acid, and l-norleucine, with a higher performance toward N-formyl-α-amino acid substrates. Biocatalyst reuse was studied, and after 10 reaction cycles, over 75 % activity remained.




Similar content being viewed by others
Notes
The pH of the solution was readjusted with NaOH after dissolution of the substrate.
The ratio of the immobilized BsLcar/GkNSAAR enzymes in terms of their activity was approximately 10:1.
Twenty-five milligrams of the A161 matrix was selected as the minimum amount to be used reproducibly.
References
Ahmad AL, Oh PC, Abd Shukor SR (2010) Synthesis of L-homophenylalanine via integrated membrane bioreactor: influence of pH on yield. Biochem Eng J 52:296–300
Altenbuchner J, Siemann-Herzberg M, Syldatk C (2001) Hydantoinases and related enzymes as biocatalysts for the synthesis of unnatural chiral amino acids. Curr Opin Biotechnol 12:559–563
Asano Y (2007) Enzymes acting on D-amino acid amides. In: Konno R, Brückner H, D’Aniello A, Fisher GH, Fujii N, Homma H (eds) D-amino acids: a new frontier in amino acid and protein research—practical methods and protocols. Nova Biomedical Books, New York, pp 579–589
Boyd WJ (1933) The isolation of amino-acids in the form of the corresponding carbamido-acids and hydantoins. Biochem J 27:1838–1848
Breuer M, Ditrich K, Habicher T, Hauer B, Kesseler M, Stürmer R, Zelinski T (2004) Industrial methods for the production of optically active intermediates. Angew Chem Int Ed Engl 43788-824
Fazary AE, Hernowo E, Angkawijaya AE, Chou T-C, Lin CH, Taha M, Ju Y-H (2011) Complex formation between ferric(III), chromium(III), and cupric(II) metal ions and (O, N) and (O, O) donor ligands with biological relevance in aqueous solution. J Solut Chem 40:1965–1986
Garcia-Galan C, Berenguer-Murcia A, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Synth Catal 353:2885–2904
Gotor V, Flitsch S (2011) Increasing the diversity of biocatalytic reactions. Curr Opin Chem Biol 15:185–186
Gutierrez M, Choi MH, Tian B, Xu J, Rho JK, Kim MO, Cho YH, Yoon SC (2013) Simultaneous inhibition of rhamnolipid and polyhydroxyalkanoic acid synthesis and biofilm formation in Pseudomonas aeruginosa by 2-bromoalkanoic acids: effect of inhibitor alkyl-chain-length. PLoS One 8:e73986. doi:10.1371/journal.pone.0073986
Hsu S, Lo H, Lin W, Chen I, Kao C, Hsu W (2007) Stereoselective synthesis of L-homophenylalanine using the carbamoylase method with in situ racemization via N-acylamino acid racemase. Process Biochem 42:856–862
Huerta FF, Minidis ABE, Bäckvall J-E (2001) Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem Soc Rev 30:321–331
Hussenet P, Le Goff P, Sennyeey G, Vincent CH (2001) Process for producing N-formylleucine of high purity. US Patent 6294692.
Johnson AL, Price WA, Wong PC, Vavala RF, Stump JM (1985) Synthesis and pharmacology of the potent angiotensin-converting enzyme inhibitor N-[1(S)-(ethoxycarbonyl)-3-phenylpropyl]-(S)-alanyl-(S)-pyroglutamic acid. J Med Chem 28:1596–1602
Kircher M (2012) How to turn industrial biotechnology into reality. New Biotechnol 29:243–247
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Liu CC, Schultz PG (2010) Adding new chemistries to the genetic code. Annu Rev Biochem 79:413–444
Martínez-Rodríguez S, Martínez-Gómez AI, Rodríguez-Vico F, Clemente-Jiménez JM, Las Heras-Vázquez FJ (2010) N-Carbamoyl-D- and L-amino acid amidohydrolases: characteristics and applications in biotechnological processes. Appl Microbiol Biotechnol 85:441–458
May O, Verseck S, Bommarius A, Drauz K (2002) Development of dynamic kinetic resolution processes for biocatalytic production of natural and nonnatural L-amino acids. Org Process Res Dev 6:452–457
Nandanwar HS, Vohra RM, Hoondal GS (2013) Enhanced stability of newly isolated trimeric L-methionine-N-carbamoylase from Brevibacillus reuszeri HSN1 by covalent immobilization. Biotechnol Appl Biochem 60:305–315
Pollegioni L, Servi S (2012) Unnatural amino acids: methods and protocols. Methods in molecular biology 794. Humana Press
Pozo-Dengra J, Martínez-Gómez AI, Martínez-Rodríguez S, Clemente-Jiménez JM, Rodríguez-Vico F, Las Heras-Vázquez FJ (2009) Racemization study on different N-acetylamino acids by a recombinant N-succinylamino acid racemase from Geobacillus kaustophilus CECT4264. Process Biochem 44:835–841
Pozo-Dengra J, Martínez-Gómez AI, Martínez-Rodríguez S, Clemente-Jiménez JM, Rodríguez-Vico F, Las Heras-Vázquez FJ (2010) Evaluation of substrate promiscuity of an L-carbamoyl amino acid amidohydrolase from Geobacillus stearothermophilus CECT43. Biotechnol Prog 26:954–959
Ragnitz K, Pietzsch M, Syldatk C (2001a) Immobilization of the hydantoin cleaving enzymes from Arthrobacter aurescens DSM 3747. J Biotechnol 92:179–186
Ragnitz K, Syldatk C, Pietzsch M (2001b) Optimization of the immobilization parameters and operational stability of immobilized hydantoinase and L-N-carbamoylase from Arthrobacter aurescens for the production of optically pure L-amino acids. Enzyme Microb Technol 28:713–720
Rodrigues RC, Ortiz C, Berenguer-Murcia Á, Torres R, Fernández-Lafuente R (2013) Modifying enzyme activity and selectivity by immobilization. Chem Soc Rev 42:6290–6307
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307
Simmons WH (2008) Thio-containing inhibitors of aminopeptidase P, and compositions thereof. US Patent 7390789:B2
Soriano-Maldonado P, Rodríguez-Alonso MJ, Hernández-Cervantes C, Rodríguez-García I, Clemente-Jiménez JM, Rodríguez Vico F, Martínez-Rodríguez S, Las Heras-Vázquez FJ (2014) Amidohydrolase process: expanding the use of L-N-carbamoylase/N-succinnyl-amino acid racemase tandem for the production of different optically pure L-amino acids. Process Biochem. doi:10.1016/j.procbio.2014.04.013
Tao R, Jiang Y, Zhu F, Yang S (2013) A one-pot system for production of L-2-aminobutyric acid from L-threonine by L-threonine deaminase and a NADH-regeneration system based on L-leucine dehydrogenase and formate dehydrogenase. Biotechnol Lett. doi:10.1007/s10529-013-1424-y
Vaidya BK, Kuwar SS, Golegaonkar SB, Nene SN (2012) Preparation of cross-linked enzyme aggregates of L-aminoacylase via co-aggregation with polyethyleneimine. J Mol Catal B Enzym 74:184–191
Yen M-C, Hsu W-H, Lin S-C (2010) Synthesis of L-homophenylalanine with immobilized enzymes. Process Biochem 45:667–674
Zhang KH, Li K, Cho M, Liao JC (2010) Expanding metabolism for total biosynthesis of the nonnatural amino acid L-homoalanine. Proc Natl Acad Sci U S A 107:6234–6239
Zhang WH, Otting G, Jackson CJ (2013) Protein engineering with unnatural amino acids. Curr Opin Struct Biol 23:581–587
Zhu L, Tao R, Wang Y, Jiang Y, Lin X, Yang Y, Zheng H, Jiang W, Yang S (2011) Removal of L-alanine from the production of L-2-aminobutyric acid by introduction of alanine racemase and D-amino acid oxidase. Appl Microbiol Biotechnol 90:903–910
Acknowledgments
This work was supported by the Spanish Ministry of Education and Science, the European Social Fund (ESF), and the European Regional Development Fund (ERDF), through project BIO2011-27842, by the Andalusian Regional Council of Innovation, Science and Technology, through project TEP-4691, and by the European Cooperation in Science and Technology (COST) Action CM1303. P.S.-M. was supported by the University of Almería. S.M.-R. was supported by the Spanish Ministry of Science and Innovation. We thank Andy Taylor for critical discussion of the manuscript and Pedro Madrid-Romero for technical assistance. We also thank Carmen Hernández-Cervantes for assistance with the polarimetry measurements.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 162 kb)
Rights and permissions
About this article
Cite this article
Soriano-Maldonado, P., Las Heras-Vazquez, F.J., Clemente-Jimenez, J.M. et al. Enzymatic dynamic kinetic resolution of racemic N-formyl- and N-carbamoyl-amino acids using immobilized l-N-carbamoylase and N-succinyl-amino acid racemase. Appl Microbiol Biotechnol 99, 283–291 (2015). https://doi.org/10.1007/s00253-014-5880-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5880-7