Abstract
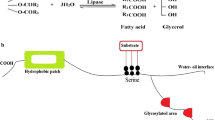
Bacterial cutinases are promising catalysts for the modification and degradation of the widely used plastic polyethylene terephthalate (PET). The improvement of the enzyme for industrial purposes is limited due to the lack of structural information for cutinases of bacterial origin. We have crystallized and structurally characterized a cutinase from Thermobifida fusca KW3 (TfCut2) in free as well as in inhibitor-bound form. Together with our analysis of the thermal stability and modelling studies, we suggest possible reasons for the outstanding thermostability in comparison to the less thermostable homolog from Thermobifida alba AHK119 and propose a model for the binding of the enzyme towards its polymeric substrate. The TfCut2 structure is the basis for the rational design of catalytically more efficient enzyme variants for the hydrolysis of PET and other synthetic polyesters.





Similar content being viewed by others
References
Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy A, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66(Pt 2):213–221. doi:10.1107/S0907444909052925
Araùjo R, Silva C, O’Neill A, Micaelo N, Guebitz G, Soares CM, Casal M, Cavaco-Paulo A (2007) Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J Biotechnol 128(4):849–857. doi:10.1016/j.jbiotec.2006.12.028
Baker PJ, Poultney C, Liu Z, Gross R, Montclare JK (2012) Identification and comparison of cutinases for synthetic polyester degradation. Appl Microbiol Biotechnol 93(1):229–240. doi:10.1007/s00253-011-3402-4
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690, http://link.aip.org/link/?JCP/81/3684/1
Bozonnet S, Jensen M, Nielsen M, Aghajari N, Jensen M, Kramhoft B, Willemoes M, Tranier S, Haser R, Svensson B (2007) The ‘pair of sugar tongs’ site on the non-catalytic domain c of barley-amylase participates in substrate binding and activity. FEBS J 274(19):5055–5067, PM:17803687
Brueckner T, Eberl A, Heumann S, Rabe M, Guebitz GM (2008) Enzymatic and chemical hydrolysis of poly(ethylene terephthalate) fabrics. J Polym Sci A Polym Chem 46(19):6435–6443. doi:10.1002/pola.22952
Burnley BT, Afonine PV, Adams PD, Gros P (2012) Modelling dynamics in protein crystal structures by ensemble refinement. Elife 1:e00311. doi:10.7554/eLife.00311
Chakravarty S, Varadarajan R (2002) Elucidation of factors responsible for enhanced thermal stability of proteins: a structural genomics based study. Biochemistry 41(25):8152–8161
Chen W, McCarthy TJ (1998) Chemical surface modification of poly(ethylene terephthalate). Macromolecules 31(11):3648–3655. doi:10.1021/ma9710601
Chen S, Tong X, Woodard RW, Du G, Wu J, Chen J (2008) Identification and characterization of bacterial cutinase. J Biol Chem 283(38):25854–25862
Chen S, Su L, Billig S, Zimmermann W, Chen J, Wu J (2010a) Biochemical characterization of the cutinases from Thermobifida fusca. J Mol Catal B Enzym 63:121–127, http://www.sciencedirect.com/science/article
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010b) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66(Pt 1):12–21. doi:10.1107/S0907444909042073
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66(Pt 4):486–501. doi:10.1107/S0907444910007493
Evans P (2006) Scaling and assessment of data quality. Acta Crystallogr D Biol Crystallogr 62(Pt 1):72–82
Greenfield NJ (2004) Analysis of circular dichroismdata. Methods Enzymol 383:282–317. doi:10.1016/S0076-6879(04)83012-X
Guebitz GM, Cavaco-Paulo A (2008) Enzymes go big: surface hydrolysis and functionalization of synthetic polymers. Trends Biotechnol 26(1):32–38. doi:10.1016/j.tibtech.2007.10.003
Haack M, Enck S, Seger H, Geyer A, Beck-Sickinger AG (2008) Pyridone dipeptide backbone scan to elucidate structural properties of a flexible peptide segment. J Am Chem Soc 130(26):8326–8336. doi:10.1021/ja8004495
Han Z-l, Han S-Y, Zheng S-Y, Lin Y (2009) Enhancing thermostability of a Rhizomucor miehei lipase by engineering a disulfide bond and displaying on the yeast cell surface. Appl Microbiol Biotechnol 85(1):117–126. doi:10.1007/s00253-009-2067-8
Herrero Acero E, Ribitsch D, Steinkellner G, Gruber K, Greimel K, Eiteljoerg I, Trotscha E, Wei R, Zimmermann W, Zinn M, Cavaco-Paulo A, Freddi G, Schwab H, Guebitz G (2011) Enzymatic surface hydrolysis of PET: effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules 44(12):4632–4640. doi:10.1021/ma200949p
Herrero Acero E, Ribitsch D, Dellacher A, Zitzenbacher S, Marold A, Steinkellner G, Gruber K, Schwab H, Guebitz GM (2013) Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol Bioeng. doi:10.1002/bit.24930
Holm L, Rosenström P (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res 38(Web Server issue):W545–W549. doi:10.1093/nar/gkq366
Hooft RW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381(6580):272. doi:10.1038/381272a0
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple amber force fields and development of improved protein backbone parameters. Proteins 65(3):712–725. doi:10.1002/prot.21123
Ikai A (1980) Thermostability and aliphatic index of globular proteins. J Biochem 88(6):1895–1898
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267(3):727–748. doi:10.1006/jmbi.1996.0897
Kabsch W (2010) XDS. Acta Crystallogr D Biol Crystallogr 66(Pt 2):125–132, PM:20124692
Kim H-W, Ishikawa K (2013) The role of disulfide bond in hyperthermophilic endocellulase. Extremophiles 17(4):593–599. doi:10.1007/s00792-013-0542-8
Kitadokoro K, Thumarat U, Nakamura R, Nishimura K, Karatani H, Suzuki H, Kawai F (2012) Crystal structure of cutinase Est119 from Thermobifida alba AHK119 that can degrade modified polyethylene terephthalate at 1.76 Å resolution. Polym Degrad Stab 97(5):771–775, http://www.sciencedirect.com/science/article
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK—a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291
Leslie AGW (1992) Recent changes to the MOSFLM package for processing film and image plate data, Joint CCP4 + ESF-EAMCB Newsletter on Proteincrystallography 26
Liu Z, Gosser Y, Baker PJ, Ravee Y, Lu Z, Alemu G, Li H, Butterfoss GL, Kong X-P, Gross R, Montclare JK (2009) Structural and functional studies of Aspergillus oryzae cutinase: enhanced thermostability and hydrolytic activity of synthetic ester and polyester degradation. J Am Chem Soc 131(43):15711–15716. doi:10.1021/ja9046697
Longhi S, Cambillau C (1999) Structure-activity of cutinase, a small lipolyticenzyme. Biochim Biophys Acta 1441(2–3):185–196
Longhi S, Czjzek M, Lamzin V, Nicolas A, Cambillau C (1997) Atomic resolution (1.0 Å) crystal structure of Fusarium solani cutinase: stereochemical analysis. J Mol Biol 268(4):779–799. doi:10.1006/jmbi.1997.1000
Murshudov GN, Skubák P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67(Pt 4):355–367. doi:10.1107/S0907444911001314
Oeser T, Wei R, Baumgarten T, Billig S, Föllner C, Zimmermann W (2010) High level expression of a hydrophobic poly(ethylene terephthalate)-hydrolyzing carboxylesterase from Thermobifida fusca KW3 in Escherichia coli BL21(DE3). J Biotechnol 146(3):100–104. doi:10.1016/j.jbiotec.2010.02.006
Porcelli M, De Leo E, Del Vecchio P, Fuccio F, Cacciapuoti G (2012) Thermal unfolding of nucleoside hydrolases from the hyperthermophilic archaeon Sulfolobus solfataricus: role of disulfidebonds. Protein Pept Lett 19(3):369–374
Riccardi C, Barni R, Selli E, Mazzone G, Massafra MR, Marcandalli B, Poletti G (2003) Surface modification of poly(ethylene terephthalate) fibers induced by radio frequency air plasma treatment. Appl Surf Sci 211:386–397, http://www.sciencedirect.com/science/article
Ronkvist M, Xie W, Lu W, Gross RA (2009) Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate). Macromolecules 42(14):5128–5138. doi:10.1021/ma9005318
Thumarat U, Nakamura R, Kawabata T, Suzuki H, Kawai F (2012) Biochemical and genetic analysis of a cutinase-type polyesterase from a thermophilic Thermobifida alba AHK119. Appl Microbiol Biotechnol 95(2):419–430. doi:10.1007/s00253-011-3781-6
Vagin A, Teplyakov A (2010) Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr 66(Pt 1):22–25
Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8(2):127–134
Wei Y, Swenson L, Castro C, Derewenda U, Minor W, Arai H, Aoki J, Inoue K, Servin-Gonzalez L, Derewenda ZS (1998) Structure of a microbial homologue of mammalian platelet-activating factor acetylhydrolases: Streptomyces exfoliatus lipase at 1.9Å resolution. Structure 6(4):511–519
Zimmermann W, Billig S (2011) Enzymes for the biofunctionalization of poly(ethylene terephthalate). Adv Biochem Eng Biotechnol 125:97–120. doi:10.1007/10_2010_87
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 517 kb)
Rights and permissions
About this article
Cite this article
Roth, C., Wei, R., Oeser, T. et al. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca . Appl Microbiol Biotechnol 98, 7815–7823 (2014). https://doi.org/10.1007/s00253-014-5672-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5672-0