Abstract
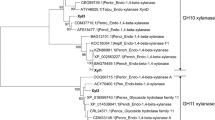
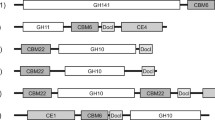
The filamentous fungus Talaromyces versatilis produces a wide range of cellulolytic and hemicellulolytic enzymes such as xylanases. The recent accessibility to the T. versatilis genome allows identifying two new genes, xynE and xynF, encoding glycoside-hydrolases from family GH11. Both genes were cloned and expressed in the methylotrophic yeast Pichia pastoris in order to compare these new xylanases with two other GH11 xylanases from T. versatilis (XynB and XynC) that were previously reported. High-level expression of recombinant enzymes was obtained for the four enzymes that were purified to homogeneity. The XynB, XynC, XynE and XynF enzymes have molecular masses of 34, 22, 45 and 23 kDa, an optimal pH between 3.5 and 4.5 and an optimal temperature between 50 °C and 60 °C. Interestingly, XynF has shown the best thermal stability at 50 °C for at least 180 min with a weak loss of activity. The four xylanases catalysed hydrolysis of low viscosity arabinoxylan (LVAX) with K m(app) between 11.5 and 23.0 mg.mL−1 and k cat/K m(app) 170 and 3,963 s−1 mg−1.mL. Further investigations on the rate and pattern of hydrolysis of the four enzymes on LVAX showed the predominant production of xylose, xylobiose and some (arabino)xylo-oligosaccharides as end products. The initial rate data from the hydrolysis of short xylo-oligosaccharides indicated that the catalytic efficiency increased with increasing degree of polymerisation of oligomer up to 6, suggesting that the specificity region of XynE and XynF spans at least six xylose residues. Because of their attractive properties, T. versatilis xylanases might be considered for biotechnological applications.





Similar content being viewed by others
References
Alcocer MJC, Furniss CSM, Kroon PA, Campbell M, Archer DB (2003) Comparison of modular and non-modular xylanases as carrier proteins for the efficient secretion of heterologous proteins from Penicillium funiculosum. Appl Microbiol Biotechnol 60:726–732. doi:10.1007/s00253-002-1184-4
Azzaz HH (2009) Effect of cellulolytic enzymes addition to diets on the productive performance of lactating goats. Thesis, Faculty of Agriculture, Cairo University, Egypt, M.Sc
Beg QK, Kapoor M, Mahajan L, Hoondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Berrin JG, Ajandouz EH, Georis J, Arnaut F, Juge N (2007) Substrate and product hydrolysis specificity in family 11 glycoside hydrolases: an analysis of Penicillium funiculosum and Penicillium griseofulvum xylanases. Appl Microbiol Biotechnol 74:1001–1010. doi:10.1007/s00253-006-0764-0
Biely P, Vrsanska M, Tenkanen M, Kluepfel D (1997) Endo-β-1,4-xylanase families: differences in catalytic properties. J Biotechnol 57:151–166
Bohlmann R, Belshaw N, Archer D, Alcocer M, Fish Neville M, Pierrard J, Guitton C (2000) Recombinant Penicillium funiculosum for homologous and heterologous protein production. Patent WO0068401
Boraston AB, Bolam DN, Gilbert HJ, Davies GJ (2004) Carbohydrate-binding modules: fine-tuning polysaccharide recognition. Biochem J 382:769–781
Brutus A, Villard C, Durand A, Tahir TA, Furniss C, Puigserver A, Juge N, Giardina T (2004) The inhibition specificity of recombinant Penicillium funiculosum xylanase B towards wheat proteinaceous inhibitors. Biochim Biophys Acta 1701:121–128. doi:10.1016/j.bbapap.2004.06.010
Buaban B, Inoue H, Yano S, Tanapongpipat S, Ruanglek V, Champreda V, Pichyangkura R, Rengpipat S, Eurwilaichitr L (2010) Bioethanol production from ball milled bagasse using an on-site produced fungal enzyme cocktail and xylose-fermenting Pichia stipitis. J Biosci Bioeng 110:18–25. doi:10.1016/j.jbiosc.2009.12.003
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238. doi:10.1093/nar/gkn663
de Castro AM, de Albuquerque de Carvalho ML, Leite SG, Pereira N Jr (2010) Cellulases from Penicillium funiculosum: production, properties and application to cellulose hydrolysis. J Ind Microbiol Biotechnol 37:151–158. doi:10.1007/s10295-009-0656-2
Cervera-Tison MC, Andre-Leroux G, Lafond M, Georis J, Juge N, Berrin J-G (2009) Molecular determinants of substrate and inhibitor specificities of the Penicillium griseofulvum family 11 xylanases. Biochim Biophys Acta 1794:438–445. doi:10.1016/j.bbapap.2008.11.024
Chavez R, Schachter K, Navarro C, Peirano A, Aguirre C, Bull P, Eyzaguirre J (2002) Differences in expresion of two endoxylanase genes (xynA and xynB) from Penicillium purpurogenum. Gene 293:161–168. doi:10.1016/S0378-1119(02)00720-5
Chavez R, Bull P, Eyzaguirre J (2006) The xylanolytic enzyme system from the genus Penicillium. J Biotechnol 123:413–433. doi:10.1016/j.jbiotec.2005.12.036
Couthino P, Henrissat B (1999) Carbohydrate-active enzymes: an integrated database approach. In: Gilbert HJ, Davies GJ, Svensson B, Henrissat B (eds) Recent advances in carbohydrate engineering. Royal Society of Chemistry, Cambridge, UK, pp 3–12
Daly R, Hearn MT (2005) Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J Mol Recognit 18:119–138. doi:10.1002/jmr.687
Dervilly G, Saulnier L, Roger P, Thibault JF (2000) Isolation of homogeneous fractions from wheat water-soluble arabinoxylans. Influence of the structure on their macromolecular characteristics. J Agric Food Chem 48:270–278. doi:10.1021/jf990222k
Driss D, Berrin JG, Juge N, Bhiri F, Ghorbel R, Chaabouni SE (2013) Functional characterization of Penicillium occitanis Pol6 and Penicillium funiculosum GH11 xylanases. Prot Expr Purif 90:195–201. doi:10.1016/j.pep.2013.06.007
Fauré R, Courtin CM, Delcour JA, Dumon C, Faulds CB, Fincher GB, Fort S, Fry SC, Halila S, Kabel MA, Pouvreau L, Quemener B, Rivet A, Saulnier L, Schols HA, Driguez H, O’Donohue MJ (2009) A brief and informationally rich naming system for oligosaccharide motifs of heteroxylans found in plant cell walls. Aus J Chem 62:533–537. doi:10.1071/ch08458
Furniss CSM, Belshaw NJ, Alcocer MJC, Williamson G, Elliott GO, Gebruers K, Haigh NP, Fish NM, Kroon PA (2002) A family 11 xylanase from Penicillium funiculosum is strongly inhibited by three wheat xylanase inhibitors. Biochim Biophys Acta 1598:24–29. doi:10.1016/S0167-4838(02)00366-7
Gilbert HJ, Knox JP, Boraston AB (2013) Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr Opin Struct Biol 23:669–677. doi:10.1016/j.sbi.2013.05.005
Gírio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101:4775–4800. doi:10.1016/j.biortech.2010.01.088
Guais O, Borderies G, Pichereaux C, Maestracci M, Neugnot V, Rossignol M, Francois JM (2008) Proteomics analysis of “RovabioTM Excel”, a secreted protein cocktail from the filamentous fungus Penicillium funiculosum grown under industrial process fermentation. J Ind Microbiol Biot 35:1659–1668. doi:10.1007/s10295-008-0430-x
Gusakov AV, Sinitsyn AP (2012) Cellulases from Penicillium species for producing fuels from biomass. Biofuels 3: 463–477. doi: 10.4155/bfs.12.41
Hewick RM, Hunkapiller MW, Hood LE, Dreyer WJ (1981) A gas–liquid solid-phase peptide and protein sequenator. J Biol Chem 256:7990–7997
Hu J, Arantes V, Saddler JN (2011) The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol Biofuels 4:36. doi:10.1186/1754-6834-4-36
Koseki T, Takahashi K, Handa T, Yamane Y, Fushinobu S, Hashizume K (2006) N-linked oligosaccharides of Aspergillus awamori feruloyl esterase are important for thermostability and catalysis. Biosci Biotechnol Biochem 70:2476–2480. doi:10.1271/bbb.60207
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–685. doi:10.1038/227680a0
Lafond M, Tauzin A, Desseaux V, Bonnin E, Ajandouz EH, Giardina T (2011) GH10 xylanase D from Penicillium funiculosum: biochemical studies and xylooligosaccharides production. Microb Cell Fact 10:20. doi:10.1186/1475-2859-10-20
Lafond M, Navarro D, Haon M, Couturier M, Berrin JG (2012) Characterization of a broad-specificity β-glucanase acting on β-(1,3)-, β-(1,4)-, and β-(1,6)-glucans that defines a new glycoside hydrolase family. Appl Environ Microbiol 78:8540–8546. doi:10.1128/AEM.02572-12
Liu G, Zhang L, Wei X, Zou G, Qin Y, Ma L, Li J, Zheng H, Wang S, Wang C, Xun L, Zhao GP, Zhou Z, Qu Y (2013) Genomic and secretomic analyses reveal unique features of the lignocellulolytic enzyme system of Penicillium decumbens. PLoS ONE 8:e55185. doi:10.1371/journal.pone.0055185
Liu L, Dong H, Wang S, Chen H, Shao W (2006) Computational analysis of di-peptides correlated with the optimal temperature in G/11 xylanase. Process Biochem 41:305–311. doi:10.1016/j.procbio.2005.06.027
Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:490–495. doi:10.1093/nar/gkt1178
Luttig M, Pretorius IS, van Zyl WH (1997) Cloning of two β-xylanase-encoding genes from Aspergillus niger and their expression in Saccharomyces cerevisiae. Biotechnol Lett 19:411–415. doi:10.1023/A:1018327623422
Madhukumar MS, Muralikrishna G (2010) Structural characterization and determination of prebiotic activity of purified xylo-oligosaccharides obtained from Bengal gram husk (Cicer arietinum L.) and wheat bran (Triticum aestivum). Food Chem 118:215–223. doi:10.1016/j.foodchem.2009.04.108
Maeda RN, Barcelos CA, Santa Anna LM, Pereira N Jr (2013) Cellulase production by Penicillium funiculosum and its application in the hydrolysis of sugar cane bagasse for second generation ethanol production by fed batch operation. J Biotechnol 163:38–44. doi:10.1016/j.jbiotec.2012.10.014
McLauchlan WR, Garcia-Conesa MT, Williamson G, Roza M, Ravestein P, Maat J (1999) A novel class of protein from wheat which inhibits xylanases. Biochem J 338:441–446. doi:10.1042/0264-6021:3380441
Moers K, Courtin CM, Brijs K, Delcour JA (2003) A screening method for endo-β-1, 4-xylanase substrate selectivity. Anal Biochem 319:73–77
Norvell LL (2011) Fungal nomenclature. 1. Melbourne approves a new Code. Mycotaxon 116:481–490. doi:10.5248/116.481
Paës G, Berrin JG, Beaugrand J (2012) GH11 xylanases: structure/function/properties relationships and applications. Biotechnol Adv 30:564–592. doi:10.1016/j.biotechadv.2011.10.003
Puchart V, Biely P (2008) Simultaneous production of endo-β-1,4-xylanase and branched xylooligosaccharides by Thermomyces lanuginosus. J Biotechnol 137:34–43. doi:10.1016/j.jbiotec.2008.07.1789
Samson RA, Yilmaz N, Houbraken J, Spierenburg H, Seifert KA, Peterson SW, Varga J, Frisvad JC (2011) Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol 70:159–183. doi:10.3114/sim.2011.70.04
Sapag A, Wouters J, Lambert C, de Joanes P, Eyzaguirre J, Depiereux E (2002) The endoxylanases from family 11: computer analysis of protein sequences reveals important structural and phylogenic relationships. J Biotechnol 95:109–131
Saulnier L, Quémener B (2009) Enzymatic mapping of arabinoxylan structure. In: Shewry PR, Ward JL (eds) Analysis of bioactive components in small grain cereals. AACC International, St Paul, Minnesota, pp 191–202
Selig MJ, Knoshaug EP, Adney WS, Himmel ME, Decker SR (2008) Synergistic enhancement of cellobiohydrolase performance on pretreated corn stover by addition of xylanase and esterase activities. Bioresour Technol 99:4997–5005. doi:10.1016/j.biortech.2007.09.064
Sims R, Taylor M, Saddler J, Mabee W (2008) From 1st- to 2nd –generation of biofuel technologies. An overview of current industry and. RD&D activities, International Energy Agency, IEA Bioenergy, Paris, France, OECD/IEA
Sunga AJ, Tolstorukov I, Cregg JM (2008) Posttransformational vector amplification in the yeast Pichia pastoris. FEMS Yeast Res 8:870–876. doi:10.1111/j.1567-1364.2008.00410.x
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol 22:33–64. doi:10.1080/07388550290789450
Van Gool MP, Van Muiswinkel GCJ, Hinz SWA, Schols HA, Sinitsyn AP, Gruppen H (2013) Two novel GH11 endo-xylanases from Myceliophthora thermophila C1 act differently toward soluble and insoluble xylans. Enz Microb Technol 53:25–32. doi:10.1016/j.enzmictec.2013.03.019
Vardakou M, Katapodis P, Samiotaki M, Kekos D, Panayotou G, Christakopoulos P (2003) Mode of action of family 10 and 11 endoxylanases on water-unextractable arabinoxylan. Int J Biol Macromol 33:129–134. doi:10.1016/S0141-8130(03)00077-1
Zhang J, Siika-Aho M, Puranen T, Tang M, Tenkanen M, Viikari L (2011) Thermostable recombinant xylanases from Nonomuraea flexuosa and Thermoascus aurantiacus show distinct properties in the hydrolysis of xylans and pretreated wheat straw. Biotechnol Biofuels 4:1–12. doi:10.1186/1754-6834-4-12
Zhang Z, Donaldson AA, Ma X (2012) Advancements and future directions in enzyme technology for biomass conversion. Biotechnol Adv 30:913–919. doi:10.1016/j.biotechadv.2012.01.020
Acknowledgments
This work was supported by grants from the CINABio-Adisseo Company (Antony, France) under the FUNZymplant project.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 1904 kb)
Rights and permissions
About this article
Cite this article
Lafond, M., Guais, O., Maestracci, M. et al. Four GH11 xylanases from the xylanolytic fungus Talaromyces versatilis act differently on (arabino)xylans. Appl Microbiol Biotechnol 98, 6339–6352 (2014). https://doi.org/10.1007/s00253-014-5606-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5606-x