Abstract
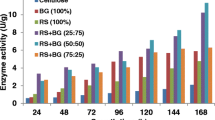
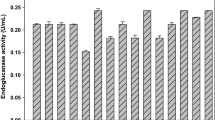
The objective of this work is to investigate the utilization of two abundant agricultural residues in Brazil for the production and application of cellulolytic enzymes. Different materials obtained after pretreatment of sugarcane bagasse, as well as pure synthetic substrates, were considered for cellulase production by Penicillium funiculosum. The best results for FPase (354 U L−1) and β-glucosidase (1,835 U L−1) production were observed when sugarcane bagasse partially delignified cellulignin (PDC) was used. The crude extract obtained from PDC fermentation was then partially characterized. Optimal temperatures for cellulase action ranged from 52 to 58°C and pH values of around 4.9 contributed to maximum enzyme activity. At 37°C, the cellulases were highly stable, losing less than 15% of their initial activity after 23 h of incubation. There was no detection of proteases in the P. funiculosum extract, but other hydrolases, such as endoxylanases, were identified (147 U L−1). Finally, when compared to commercial preparations, the cellulolytic complex from P. funiculosum showed more well-balanced amounts of β-glucosidase, endo- and exoglucanase, resulting in the desired performance in the presence of a lignocellulosic material. Cellulases from this filamentous fungus had a higher glucose production rate (470 mg L−1 h−1) when incubated with corn cob than with Celluclast®, GC 220® and Spezyme® (312, 454 and 400 mg L−1 h−1, respectively).





Similar content being viewed by others
References
IBGE (2008) SIDRA-Sistema de Recuperação Automática. http://www.sidra.ibge.gov.br, accessed Nov 2008
CONAB (2008) Companhia Nacional de Abastecimento. http://www.conab.gov.br, accessed Nov 2008
Carvalho FC (1992) Disponibilidade de resíduos agroindustriais e do beneficiamento de produtos agrícolas. Informações Econômicas 22(12):31–46
FAOSTAT (2008) Homepage. http://faostat.fao.org, accessed Nov 2008
BNDES (2007) Ampliação da produção de etanol e co-geração de energia elétrica. http://www.bndes.gov.br/conhecimento/seminario/alcool_discussao.pdf, accessed Oct 2007
SUN JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Pol Degr Stab 84:331–339. doi:10.1016/j.polymdegradstab.2004.02.008
Zhang YP, Lynd LR (2004) Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulase systems. Biotechnol Bioeng 88(7):797–824. doi:10.1002/bit.20282
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–577. doi:10.1128/MMBR.66.3.506-577.2002
Camassola M, Bittencourt RS, Shenen NT, Andreaus J, Dillon AJP (2004) Characterization of the cellulase complex of Penicillium echinulatum. Biocat Biotrans 22(5/6):391–396. doi:10.1080/10242420400024532
Jorgensen H, Eriksson T, Borjesson J, Tjerneld F, Olsson L (2003) Purification and characterization of five cellulases and one xylanase from Penicillium brasilianum IBT 20888. Enzyme Microb Technol 32:851–861. doi:10.1016/j.enzmictec.2005.06.018
Jorgensen H, Morkeberg A, Krogh KBR, Olsson L (2005) Production of cellulases and hemicellulases by three Penicillium species: effect of substrate and evaluation of cellulase adsorption by capillary electrophoresis. Enzyme Microb Technol 36:42–48. doi:10.1016/j.enzmictec.2005.06.018
Jorgensen H, Olsson L (2006) Production of cellulases by Penicillium brasilianum IBT 20888–Effect of substrate on hydrolytic performance. Enzyme Microb Technol 38(3–4):381–390. doi:10.1016/j.enzmictec.2005.06.018
Krogh KBR, Morkeberg A, Jorgensen H, Frisvad JC, Olsson L (2004) Screening genus Penicillium for producers of cellulolytic and xylanolytic enzymes. Appl Biochem Biotechnol 113–116:389–401. doi:10.1385/ABAB:114:1-3:389
Van Wyk JPH (1999) Saccharification of paper products by cellulase from Penicillium funiculosum and Trichoderma reesei. Biom Bioen 16:239–242. doi:10.1016/S0961-9534(98)00079-8
Bhat MK (2000) Cellulase and related enzymes in biotechnology. Biotechnol Adv 18:355–383. doi:10.1016/S0734-9750(00)00041-0
Godfrey T, West S (1996) Industrial enzymology, 2nd edn. Macmillan, London
Tolan JS, Foody B (1999) Cellulase from submerged fermentation. Adv Biochem Eng Biotechnol 65:41–67. doi:10.1007/3-540-49194-5_3
Kaar WE, Cool LG, Merriman MM, Brink DL (1991) The complete analysis of wood polysaccharides using HPLC. J Wood Chem Techn 11:447. doi:10.1080/02773819108051086
Szijártó N, Szengyel Z, Lidén G, Réczey K (2004) Dynamics of cellulase production by glucose grown cultures of Trichoderma reesei Rut-C30 as a response to addition of cellulose. Appl Biochem Biotechnol 113–116:115–124. doi:10.1385/ABAB
Ghose TK (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270. doi:10.1016/0168-1656(92)90074-J
Charney J, Tomarelli RM (1947) A colorimetric method for the determination of the proteolytic activity of duodenal juice. J Biol Chem 171(2):501–505
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Mo H, Zhang X, Li Z (2004) Control of gas phase for enhanced cellulase production by Penicillium decumbens in solid-state culture. Process Biochem 39:1293–1297. doi:10.1016/S0032-9592(03)00291-7
Aiello C, Ferrer A, Ledesma A (1996) Effect of alkaline treatments at various temperatures on cellulase and biomass production using submerged sugarcane bagasse fermentation with Trichoderma reesei QM 9414. Bioresour Technol 57:13–18. doi:10.1016/0960-8524(96)00012-0
Camassola M, Dillon AJP (2009) Biological pretreatment of sugar cane bagasse for the production of cellulases and xylanases by Penicillium echinulatum. Ind Crops Prod 29:642–647. doi:10.1016/j.indcrop.2008.09.008
Kádár Z, Szengyel Z, Réczey K (2004) Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind Crop Prod 20:103–110. doi:10.1016/j.indcrop.2003.12.015
Fujita Y, Ito J, Ueda M, Fukuda H, Kondo A (2004) Syneristic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of and engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl Environ Microbiol 70(2):1207–1212. doi:10.1128/AEM.70.2.1207-1212.2004
Tanaka T, Hoshina M, Tanabe S, Sakai K, Ohtsubo S, Taniguchi M (2006) Production of D-lactic acid from defatted rice bran by simultaneous saccharification and fermentation. Bioresour Technol 97(2):211–217. doi:10.1016/j.biortech.2005.02.025
Cavaco-Paulo A (1998) Mechanism of cellulase action in textile processes. Carbohydr Polym 37:273–277. doi:10.1016/S0144-8617(98)00070-8
Acknowledgments
To Dr. Alexandre Soares dos Santos, Marcela C. Ferreira, Juliana C. Cruz, Daniele F. Carvalho, Kelly C. N. R. Pedro and Roberto Maeda, for their technical assistance. We are also grateful to the Brazilian Council for Research (CNPq), the Rio de Janeiro State Foundation for Science and Technology (FAPERJ), and the Brazilian Petroleum Company (PETROBRAS) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Castro, A.M., de Albuquerque de Carvalho, M.L., Leite, S.G.F. et al. Cellulases from Penicillium funiculosum: production, properties and application to cellulose hydrolysis. J Ind Microbiol Biotechnol 37, 151–158 (2010). https://doi.org/10.1007/s10295-009-0656-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-009-0656-2