Abstract
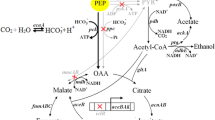
Although many efforts had been performed to engineer Escherichia coli for succinate production, succinate efflux system had not been investigated as an engineering target for improving succinate production. In this work, four Dcu transporters, which had been reported to be responsible for C4-dicarboxylates transportation of E. coli, were investigated for their succinate efflux capabilities. These four dcu genes were deleted individually in a previously constructed succinate-producing strain to study their effects on succinate production. Deleting dcuA and dcuD genes had nearly no influence, while deleting dcuB and dcuC genes led to 15 and 11 % decrease of succinate titer, respectively. Deleting both dcuB and dcuC genes resulted in 90 % decrease of succinate titer, suggesting that DcuB and DcuC were the main transporters for succinate efflux and they functioned as independent and mutually redundant succinate efflux transporters. Furthermore, RBS library having strengths varied from 0.17 to 8.6 times of induced E. coli lacZ promoter was used to modulate dcuB and dcuC genes for improving succinate production. Modulating these two genes in combination led to 34 % increase of succinate titer. To the best of knowledge, this was the first report about improving succinate production through engineering succinate efflux system.




Similar content being viewed by others
References
Carrier TA, Keasling JD (1999) Library of synthetic 5′ secondary structures to manipulate mRNA stability in Escherichia coli. Biotechnol Prog 15:58–64
Chatterjee R, Millard CS, Champion K, Clark DP, Donnelly MI (2001) Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl Environ Microbiol 67:148–154
Golby P, Kelly DJ, Guest JR, Andrews SC (1998) Transcriptional regulation and organization of the dcuA and dcuB genes, encoding homologous anaerobic C4-dicarboxylate transporters in Escherichia coli. J Bacteriol 180:6586–6596
Janausch IG, Unden G (1999) The dcuD (former yhcL) gene product of Escherichia coli as a member of the DcuC family of C4-dicarboxylate carriers: lack of evident expression. Arch Microbiol 172:219–226
Janausch IG, Kim OB, Unden G (2001) DctA- and Dcu-independent transport of succinate in Escherichia coli: contribution of diffusion and of alternative carriers. Arch Microbiol 176:224–230
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008a) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99:1140–1153
Jantama K, Zhang X, Moore JC, Shanmugam KT, Svoronos SA, Ingram LO (2008b) Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol Bioeng 101:881–893
Lee SJ, Lee DY, Kim TY, Kim BH, Lee J, Lee SY (2005) Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl Environ Microbiol 71:7880–7887
Lin H, Bennett GN, San KY (2005) Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol Bioeng 90:775–779
Lu J, Tang J, Liu Y, Zhu X, Zhang T, Zhang X (2012) Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol 93:2455–2462
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740
Miller JH (1992) A short course in bacterial genetics. Cold Spring Harbor Laboratory, New York, pp 71–74
Shi A, Zhu X, Lu J, Zhang X, Ma Y (2013) Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10
Singh A, Cher Soh K, Hatzimanikatis V, Gill RT (2011) Manipulating redox and ATP balancing for improved production of succinate in E. coli. Metab Eng 13:76–81
Six S, Andrews SC, Unden G, Guest JR (1994) Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct). J Bacteriol 176:6470–6478
Stols L, Donnelly MI (1997) Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol 63:2695–2701
Tan Z, Zhu X, Chen J, Li Q, Zhang X (2013) Activating phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase in combination for improvement of succinate production. Appl Environ Microbiol 79:4838–4844
Tang J, Zhu X, Lu J, Liu P, Xu H, Tan Z, Zhang X (2013) Recruiting alternative glucose utilization pathways for improving succinate production. Appl Microbiol Biotechnol 97:2513–2520
Wang Q, Wu C, Chen T, Chen X, Zhao X (2006) Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions. Biotechnol Lett 28:89–93
Zhang X, Jantama K, Moore JC, Shanmugam KT, Ingram LO (2007) Production of l-alanine by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77:355–366
Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO (2009a) Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci U S A 106:20180–20185
Zhang X, Jantama K, Shanmugam KT, Ingram LO (2009b) Reengineering Escherichia coli for succinate production in mineral salts medium. Appl Environ Microbiol 75:7807–7813
Zhao J, Li Q, Sun T, Zhu X, Xu H, Tang J, Zhang X, Ma Y (2013) Engineering central metabolic modules of Escherichia coli for improving beta-carotene production. Metab Eng 17:42–50
Zientz E, Janausch IG, Six S, Unden G (1999) Functioning of DcuC as the C4-dicarboxylate carrier during glucose fermentation by Escherichia coli. J Bacteriol 181:3716–3720
Acknowledgments
This research was supported by grants from the Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-G-2), the National Basic Research Program of China (2011CBA00800), and the National High Technology Research and Development Program of China (2011AA02A203). Xueli Zhang was supported by the Hundred Talent Program of the Chinese Academy of Sciences.
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Chen and Xinna Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, J., Zhu, X., Tan, Z. et al. Activating C4-dicarboxylate transporters DcuB and DcuC for improving succinate production. Appl Microbiol Biotechnol 98, 2197–2205 (2014). https://doi.org/10.1007/s00253-013-5387-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5387-7