Abstract
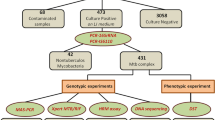
Rapid detection of drug-resistant Mycobacterium tuberculosis is critical to the effective early treatment and prevention of the transmission of tuberculosis. However, conventional drug susceptibility tests for M. tuberculosis require up to several weeks. In the present study, the One Label Extension genotyping method was adapted for rapid detection of drug resistance-associated sequence variations in six genes of M. tuberculosis, viz. rpoB, rpsL, rrs, embB, katG, or inhA. The method utilizes polymerase chain reaction amplified fragments of the drug resistant genes as reaction templates, and proceeds with template-directed primer extension incorporating a fluorescence-labeled nucleotide, which is then measured by fluorescence polarization. A total of 121 M. tuberculosis isolates from clinical sputum specimens were examined by this genotyping method and verified by direct sequencing of polymerase chain reaction amplicons harboring previously reported mutational sites associated with M. tuberculosis drug resistance. Based on phenotyping results obtained from microbiology-based drug susceptibility tests, the sensitivity, specificity, and test efficiency estimated for One Label Extension assays were respectively 83.9 %, 95.5 %, and 92.4 % with ropB in rifampin resistance, 67.3 %, 97.1 %, and 84.3 % with rpsL and rrs in streptomycin resistance, 60.0 %, 96.0 %, and 91.4 % with embB in ethambutol resistance, 68.4 %, 94.9 %, and 86.3 % with inhA and katG in isoniazid resistance, and 74.1 %, 98.9 %, and 93.2 % in multiple drug resistance defined as resistance to at least both isoniazid and rifampin. In conclusion, examination of clinical sputum specimens by One Label Extension based genotyping provides a valid method for the rapid molecular detection of drug-resistant M. tuberculosis.


Similar content being viewed by others
References
Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, Collins D, de Lisle G, Jacobs WR Jr (1994) inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263(5144):227–230. doi:10.1126/science.8284673
Brossier F, Veziris N, Aubry A, Jarlier V, Sougakoff W (2010) Detection by GenoType MTBDRsl test of complex mechanisms of resistance to second-line drugs and ethambutol in multidrug-resistant Mycobacterium tuberculosis complex isolates. J Clin Microbiol 48(5):1683–1689. doi:10.1128/JCM.01947-09
Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, Rist N, Smelev NA (1969) Advances in techiques of testing myobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull W H O 41:21–43
Caws M, Drobniewski FA (2001) Molecular techniques in the diagnosis of Mycobacterium tuberculosis and the detection of drug resistance. Ann NY Acad Sci 953:138–145. doi:10.1111/j.1749-6632.2001.tb11371.x
Choi JH, Lee KW, Kang HR, Hwang YI, Jang S, Kim DG, Kim CH, Hyun IG, Shin TR, Park SM, Lee MG, Lee CY, Park YB, Jung KS (2010) Clinical efficacy of direct DNA sequencing analysis on sputum specimens for early detection of drug-resistant Mycobacterium tuberculosis in a clinical setting. Chest 137(2):393–400. doi:10.1378/chest.09-0150
Engström A, Morcillo N, Imperiale B, Hoffner SE, Juréen P (2012) Detection of first- and second-line drug resistance in Mycobacterium tuberculosis clinical isolates by pyrosequencing. J Clin Microbiol 50(6):2026–2033. doi:10.1128/JCM.06664-11
Georghiou SB, Magana M, Garfein RS, Catanzaro DG, Catanzaro A, Rodwell TC (2012) Evaluation of genetic mutations associated with Mycobacterium tuberculosis resistance to amikacin, kanamycin and capreomycin: a systematic review. PLoS One 7(3):e33275. doi:10.1371/journal.pone.0033275
Huang WL, Chi TL, Wu MH, Jou R (2011) Performance assessment of the GenoType MTBDRsl test and DNA sequencing for detection of second-line and ethambutol drug resistance among patients infected with multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 49(7):2502–2508. doi:10.1128/JCM.00197-11
Kapur V, Li LL, Iordanescu S, Hamrick MR, Wanger A, Kreiswirth BN, Musser JM (1994) Characterization by automated DNA sequencing of mutations in the gene (rpoB) encoding the RNA polymerase β subunit in rifampin-resistant Mycobacterium tuberculosis strains from New York City and Texas. J Clin Microbiol 32(4):1095–1098
Lawn SD, Nicol MP (2011) Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol 6(9):1067–1082. doi:10.2217/fmb.11.84
Lee AS, Ong DC, Wong JC, Siu GK, Yam WC (2012) High-resolution melting analysis for the rapid detection of fluoroquinolone and streptomycin resistance in Mycobacterium tuberculosis. PLoS One 7(2):e31934. doi:10.1371/journal.pone.0031934
Li J, Xin J, Zhang L, Jiang L, Cao H, Li L (2012) Rapid detection of rpoB mutations in rifampin resistant M. tuberculosis from sputum samples by denaturing gradient gel electrophoresis. Int J Med Sci 9(2):148–156. doi:10.7150/ijms.3605
Ling DI, Zwerling AA, Pai M (2008) GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J 32(5):1165–1174. doi:10.1183/09031936.00061808
Lipin MY, Stepanshina VN, Shemyakin IG, Shinnick TM (2007) Association of specific mutations in katG, rpoB, rpsL and rrs genes with spoligotypes of multidrug-resistant Mycobacterium tuberculosis isolates in Russia. Clin Microbiol Infect 13(6):620–626. doi:10.1111/j.1469-0691.2007.01711.x
Marín M, García de Viedma D, Ruíz-Serrano MJ, Bouza E (2004) Rapid direct detection of multiple rifampin and isoniazid resistance mutations in Mycobacterium tuberculosis in respiratory samples by real-time PCR. Antimicrob Agents Chemother 48(11):4293–4300. doi:10.1128/AAC.48.11.4293-4300.2004
Mokrousov I, Bhanu NV, Suffys PN, Kadival GV, Yap SF, Cho SN, Jordaan AM, Narvskaya O, Singh UB, Gomes HM, Lee H, Kulkarni SP, Lim KC, Khan BK, van Soolingen D, Victor TC, Schouls LM (2004) Multicenter evaluation of reverse line blot assay for detection of drug resistance in Mycobacterium tuberculosis clinical isolates. J Microbiol Methods 57(3):323–335. doi:10.1016/j.mimet.2004.02.006
Newcombe RG (1998) Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 17(8):857–872. doi:10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E
Nickerson DA, Tobe VO, Taylor SL (1997) PolyPhred: automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res 25(14):2745–2751. doi:10.1093/nar/25.14.2745
Nicol MP (2010) New developments in the laboratory diagnosis of tuberculosis. Continu Med Educ 28:246–250
O’Grady J, Maeurer M, Mwaba P, Kapata N, Bates M, Hoelscher M, Zumla A (2011) New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr Opin Pulm Med 17(3):134–141. doi:10.1097/MCP.0b013e3283452346
Pérez-Osorio AC, Boyle DS, Ingham ZK, Ostash A, Gautom RK, Colombel C, Houze Y, Leader BT (2012) Rapid identification of mycobacteria and drug-resistant Mycobacterium tuberculosis by use of a single multiplex PCR and DNA sequencing. J Clin Microbiol 50(2):326–336. doi:10.1128/JCM.05570-11
Pai M, Minion J, Sohn H, Zwerling A, Perkins MD (2009) Novel and improved technologies for tuberculosis diagnosis: progress and challenges. Clin Chest Med 30(4):701–716. doi:10.1016/j.ccm.2009.08.016, viii
Plinke C, Walter K, Aly S, Ehlers S, Niemann S (2011) Mycobacterium tuberculosis embB codon 306 mutations confer moderately increased resistance to ethambutol in vitro and in vivo. Antimicrob Agents Chemother 55(6):2891–2896. doi:10.1128/AAC.00007-10
Ramasubban G, Therese KL, Vetrivel U, Sivashanmugam M, Rajan P, Sridhar R, Madhavan HN, Meenakshi N (2011) Detection of novel coupled mutations in the katG gene (His276Met, Gln295His and Ser315Thr) in a multidrug-resistant Mycobacterium tuberculosis strain from Chennai, India, and insight into the molecular mechanism of isoniazid resistance using structural bioinformatics approaches. Int J Antimicrob Agents 37(4):368–372. doi:10.1016/j.ijantimicag.2010.11.023
Roberts GD, Goodman NL, Heifets L, Larsh HW, Lindner TH, McClatchy JK, McGinnis MR, Siddiqi SH, Wright P (1983) Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol 18(3):689–696
Safi H, Sayers B, Hazbón MH, Alland D (2008) Transfer of embB codon 306 mutations into clinical Mycobacterium tuberculosis strains alters susceptibility to ethambutol, isoniazid, and rifampin. Antimicrob Agents Chemother 52(6):2027–2034. doi:10.1128/AAC.01486-07
Schön T, Juréen P, Giske CG, Chryssanthou E, Sturegård E, Werngren J, Kahlmeter G, Hoffner SE, Angeby KA (2009) Evaluation of wild-type MIC distributions as a tool for determination of clinical breakpoints for Mycobacterium tuberculosis. J Antimicrob Chemother 64(4):786–793. doi:10.1093/jac/dkp262
Sekiguchi J, Miyoshi-Akiyama T, Augustynowicz-Kopeć E, Zwolska Z, Kirikae F, Toyota E, Kobayashi I, Morita K, Kudo K, Kato S, Kuratsuji T, Mori T, Kirikae T (2007) Detection of multidrug resistance in Mycobacterium tuberculosis. J Clin Microbiol 45(1):179–192. doi:10.1128/JCM.00750-06
Spies FS, von Groll A, Ribeiro AW, Ramos DF, Ribeiro MO, Dalla Costa ER, Martin A, Palomino JC, Rossetti ML, Zaha A, da Silva PE (2013) Biological cost in Mycobacterium tuberculosis with mutations in the rpsL, rrs, rpoB, and katG genes. Tuberculosis (Edinb) 93(2):150–154. doi:10.1016/j.tube.2012.11.004
Sreevatsan S, Pan X, Stockbauer KE, Williams DL, Kreiswirth BN, Musser JM (1996) Characterization of rpsL and rrs mutations in streptomycin-resistant Mycobacterium tuberculosis isolates from diverse geographic localities. Antimicrob Agents Chemother 40(4):1024–1026
Sreevatsan S, Stockbauer KE, Pan X, Kreiswirth BN, Moghazeh SL, Jacobs WR Jr, Telenti A, Musser JM (1997) Ethambutol resistance in Mycobacterium tuberculosis: critical role of embB mutations. Antimicrob Agents Chemother 41(8):1677–1681
Tang X, Morris SL, Langone JJ, Bockstahler LE (2005) Microarray and allele specific PCR detection of point mutations in Mycobacterium tuberculosis genes associated with drug resistance. J Microbiol Methods 63(3):318–330. doi:10.1016/j.mimet.2005.04.026
Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, Matter L, Schopfer K, Bodmer T (1993) Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 341(8846):647–650. doi:10.1016/0140-6736(93)90417-F
Tracevska T, Jansone I, Nodieva A, Marga O, Skenders G, Baumanis V (2004) Characterisation of rpsL, rrs and embB mutations associated with streptomycin and ethambutol resistance in Mycobacterium tuberculosis. Res Microbiol 155(10):830–834. doi:10.1016/j.resmic.2004.06.007
Van Rie A, Page-Shipp L, Scott L, Sanne I, Stevens W (2010) Xpert® MTB/RIF for point-of-care diagnosis of TB in high-HIV burden, resource-limited countries: hype or hope? Expert Rev Mol Diagn 10(7):937–946. doi:10.1586/erm.10.67
WHO (1998a) Laboratory services in tuberculosis control. Part II: microscopy. WHO/TB/98.258. World Heath Organization, Geneva, Switzerland. http://www.who.int/tb/publications/who_tb_98_258/en/
WHO (1998b) Laboratory services in tuberculosis control. Part III: culture. WHO/TB/98.258. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/who_tb_98_258/en/
WHO (2008a) Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB). World Health Organization, Geneva, Switzerland. http://www.who.int/tb/features_archive/policy_statement.pdf
WHO (2008b) Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. WHO/HTM/TB/2008.392. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/2008/whohtmtb_2008_392/en/
WHO (2008c) WHO report 2008. Global tuberculosis control: surveillance, planning, financing. WHO/HTM/TB/2008.393. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_report/2008/en/
WHO (2010) Tuberculosis diagnostics automated DNA test. WHO endorsement and recommendations. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/features_archive/xpert_factsheet.pdf
Yu Z, Chen J, Shi H, Stoeber G, Tsang SY, Xue H (2006) Analysis of GABRB2 association with schizophrenia in German population with DNA sequencing and one-label extension method for SNP genotyping. Clin Biochem 39(3):210–218. doi:10.1016/j.clinbiochem.2006.01.009
Zhang Y, Heym B, Allen B, Young D, Cole S (1992) The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358(6387):591–593. doi:10.1038/358591a0
Acknowledgments
We thank Mr. Ka-King Chu and Ms. Peggy Lee for technical assistance. Financial support from the Pharmacogenetics Limited of Hong Kong, the National Health and Family Planning Commission (grant no. 2013ZX10003001), and Department of Science and Information Technology of Guangzhou (grant no. 2013J4500012), P. R. China are gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yisuo Sun, Shufen Li, and Lin Zhou contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sun, Y., Li, S., Zhou, L. et al. A rapid fluorescence polarization-based method for genotypic detection of drug resistance in Mycobacterium tuberculosis . Appl Microbiol Biotechnol 98, 4095–4105 (2014). https://doi.org/10.1007/s00253-013-5356-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5356-1