Abstract
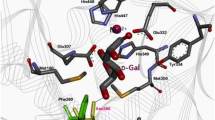
l-Arabinose isomerase (l-AI) catalyzes the isomerization of l-arabinose to l-ribulose and d-galactose to d-tagatose. Most reported l-AIs exhibit neutral or alkaline optimum pH, which is less beneficial than acidophilic ones in industrial d-tagatose production. Lactobacillus fermentum l-AI (LFAI) is a thermostable enzyme that can achieve a high conversion rate for d-galactose isomerization. However, its biocatalytic activity at acidic conditions can still be further improved. In this study, we report the single- and multiple-site mutagenesis on LFAI targeting three aspartic acid residues (D268, D269, and D299). Some of the lysine mutants, especially D268K/D269K/D299K, exhibited significant optimum pH shifts (from 6.5 to 5.0) and enhancement of pH stability (half-life time increased from 30 to 62 h at pH 6.0), which are more favorable for industrial applications. With the addition of borate, d-galactose was isomerized into d-tagatose by D268K/D269K/D299K at pH 5.0, resulting in a high conversion rate of 62 %. Based on the obtained 3.2-Å crystal structure of LFAI, the three aspartic acid residues were found to be distant from the active site and possibly did not participate in substrate catalysis. However, they were proven to possess similar optimum pH control ability in other l-AI, such as that derived from Escherichia coli. This study sheds light on the essential residues of l-AIs that can be modified for desired optimum pH and better pH stability, which are useful in d-tagatose bioproduction.







Similar content being viewed by others
References
Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D-Biol Crystallogr 66(2):213–221
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cheetham PSJ, Wootton AN (1993) Bioconversion of d-galactose into d-tagatose. Enzyme Microb Technol 15:105–108
Cheng L, Mu W, Zhang T, Jiang B (2010) An l-arabinose isomerase from Acidothermus cellulolytics ATCC 43068: cloning, expression, purification, and characterization. Appl Microbiol Biotechnol 86(4):1089–1097
Chouayekh H, Bejar W, Rhimi M, Jelleli K, Mseddi M, Bejar S (2007) Characterization of an l-arabinose isomerase from the Lactobacillus plantarum NC8 strain showing pronounced stability at acidic pH. FEMS Microbiol Lett 277:260–267
Dische Z, Borenfreund E (1951) A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem 192:583–587
DeLano WL (2002) The PyMOL molecular graphics system. DeLano Scientific, San Carlos, CA, USA. http://www.pymol.org
Donner TW, Wilber JF, Ostrowski D (1999) d-Tagatose, a novel hexose: acute effects on carbohydrate tolerance in subjects with and without type 2 diabetes. Diabetes Obes Metab 1:285–291
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D-Biol Crystallogr 66(4):486–501
Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R (2010) Concept of vulnerable/unstable plaque. Arterioscl Throm Vas 30(7):1282–1292
FDA (2003) Food labeling: health claims; d-tagatose and dental caries. Final rule http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12848171
Jørgensen F, Hansen OC, Stougaard P (2004) Enzymatic conversion of d-galactose to d-tagatose: heterologous expression and characterization of a thermostable l-arabinose isomerase from Thermoanaerobacter mathranii. Appl Microbiol Biotechnol 64:816–822
Kim BC, Lee YH, Lee HS, Lee DW, Choe EA, Pyun YR (2002) Cloning, expression and characterization of l-arabinose isomerase from Thermotoga neapolitana: bioconversion of d-galactose to d-tagatose using the enzyme. FEMS Microbiol Lett 212(1):121–126
Kim HJ, Oh DK (2005) Purification and characterization of an l-arabinose isomerase from an isolated strain of Geobacillus thermodenitrificans producing d-tagatose. J Biotechnol 120(2):162–173
Levin GV (2002) Tagatose, the new GRAS sweetener and health product. J Med Food 5:23–36
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck - a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Li L, Li C, Sarkar S, Zhang J, Witham S, Zhang Z, Wang L, Smith N, Petukh M, Alexov E (2012) DelPhi: a comprehensive suite for DelPhi software and associated resources. BMC Biophys 5(1):9
Lim BC, Kim HJ, Oh DK (2007) High production of d-tagatose by the addition of boric acid. Biotechnol Prog 23:824–828
Lee DW, Jang HJ, Choe EA, Kim BC, Lee SJ, Kim SB, Hong YH, Pyun YR (2004) Characterization of a thermostable l-arabinose (d-galactose) isomerase from the hyperthermophilic eubacterium Thermotoga maritima. Appl Environ Microbiol 70:1397–1404
Lee SJ, Lee DW, Choe EA, Hong YH, Kim SB, Kim BC, Pyun YR (2005) Characterization of a thermoacidophilic l-arabinose isomerase from Alicyclobacillus acidocaldarius: role of Lys-269 in pH optimum. Appl Environ Microbiol 71:7888–7896
Manjasetty BA, Chance MR (2006) Crystal structure of Escherichia coli l-arabinose isomerase (ECAI), the putative target of biological tagatose production. J Mol Biol 360:297–309
Mccoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) Phaser crystallographic software. J Appl Crystallogr 40:658–674
Oh DK, Oh HJ, Kim HJ, Cheon J, Kim P (2006) Modification of optimal pH in l-arabinose isomerase from Geobacillus stearothermophilus for d-galactose isomerization. J Mol Catal B Enzym 43:108–112
Oh DK (2007) Tagatose: properties, applications, and biotechnological processes. Appl Microbiol Biotechnol 76:1–8
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326
Rhimi M, Bejar S (2006) Cloning, purification and biochemical characterization of metallic-ions independent and thermoactive l-arabinose isomerase from the Bacillus stearothermophilus US100 strain. Biochim Biophys Acta 1760:191–199
Rhimi M, Aghajari N, Juy M, Chouayekh H, Maguin E, Haser R, Bejar S (2009) Rational design of Bacillus stearothermophilus US100 l-arabinose isomerase: potential applications for d-tagatose production. Biochimie 91(5):650–653
Rhimi M, Ihammami R, Bajic G, Boudebbouze S, Maguin E, Haser R, Aghajari N (2010) The acid tolerant l-arabinose isomerase from the food grade Lactobacillus sakei 23K is an attractive d-tagatose producer. Bioresour Technol 101:9171–9177
Rhimi M, Bajic G, Ilhammami R, Boudebbouze S, Maguin E, Haser R, Aghajari N (2011a) The acid-tolerant l-arabinose isomerase from the mesophilic Shewanella sp. ANA-3 is highly active at low temperatures. Microb Cell Fact 10:96
Rhimi M, Chouayekh H, Gouillouard I, Maguin E, Bejar S (2011b) Production of d-tagatose, a low caloric sweetener during milk fermentation using l-arabinose isomerase. Bioresour Technol 102:3309–3315
Ryu SA, Kim CS, Kim HJ, Baek DH, Oh DK (2003) Continuous d-tagatose production by immobilized thermostable l-arabinose isomerase in a packed-bed bioreactor. Biotechnol Prog 19:1643–1647
Vagin AA, Steiner RA, Lebedev AA, Potterton L, McNicholas S, Long F, Murshudov GN (2004) REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D-Biol Cryst 60(12):2184–2195
Xu Z, Qing YJ, Li S, Feng XH, Xu H, Ouyang PK (2011) A novel l-arabinose isomerase from Lactobacillus fermentum CGMCC2921 for d-tagatose production: gene cloning, purification and characterization. J Mol Catal B Enzym 70:1–7
Xu Z, Li S, Fu FG, Li GX, Feng X, Xu H, Ouyang PK (2012) Production of d-tagatose, a functional sweetener, utilizing alginate immobilized Lactobacillus fermentum CGMCC2921 cells. Appl Biochem Biotechnol 166:961–973
Acknowledgements
This work was supported by National Basic Research Program of China (2013CB733600), the National High Technology Research and Development Program of China (2012AA021503), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (IRT1066), Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP) (20113221130001), the National Natural Science Foundation of China (31371732).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3124 kb)
Rights and permissions
About this article
Cite this article
Xu, Z., Li, S., Feng, X. et al. Function of aspartic acid residues in optimum pH control of l-arabinose isomerase from Lactobacillus fermentum . Appl Microbiol Biotechnol 98, 3987–3996 (2014). https://doi.org/10.1007/s00253-013-5342-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5342-7