Abstract
Fatty acids are a promising raw material for substance production because of their highly reduced and anhydrous nature, which can provide higher fermentation yields than sugars. However, they are insoluble in water and are poorly utilized by microbes in industrial fermentation production. We used fatty acids as raw materials for l-lysine fermentation by emulsification and improved the limited fatty acid-utilization ability of Escherichia coli. We obtained a fatty acid-utilizing mutant strain by laboratory evolution and demonstrated that it expressed lower levels of an oxidative-stress marker than wild type. The intracellular hydrogen peroxide (H2O2) concentration of a fatty acid-utilizing wild-type E. coli strain was higher than that of a glucose-utilizing wild-type E. coli strain. The novel mutation rpsA D210Y identified in our fatty acid-utilizing mutant strain enabled us to promote cell growth, fatty-acid utilization, and l-lysine production from fatty acid. Introduction of this rpsA D210Y mutation into a wild-type strain resulted in lower H2O2 concentrations. The overexpression of superoxide dismutase (sodA) increased intracellular H2O2 concentrations and inhibited E. coli fatty-acid utilization, whereas overexpression of an oxidative-stress regulator (oxyS) decreased intracellular H2O2 concentrations and promoted E. coli fatty acid utilization and l-lysine production. Addition of the reactive oxygen species (ROS) scavenger thiourea promoted l-lysine production from fatty acids and decreased intracellular H2O2 concentrations. Among the ROS generated by fatty-acid β-oxidation, H2O2 critically affected E. coli growth and l-lysine production. This indicates that the regression of ROS stress promotes fatty acid utilization, which is beneficial for fatty acids used as raw materials in industrial production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acids are stored as triglycerides within organisms and are an important source of energy as they are both highly reduced and anhydrous. Indeed, the energy yield from 1 g fatty acid is more than twice that from carbohydrate. However, industrial production by fermentation mainly uses sugars such as glucose and sucrose as raw materials, as fatty acids are insoluble in water and are poorly utilized by producer strains. Recently, biodiesel production from microalgae as a renewable energy source has received considerable attention (Chisti 2007). Commercial microalgae cultures for fatty acid production and the bioconversion of fatty acids to fuels and chemicals by microorganisms are attractive alternative carbon resources for substance production (Chen et al. 2011; Dellomonaco et al. 2010; Hu et al. 2008; Rosenberg et al. 2008; Service 2009). High-yield aerobic fermentation by Escherichia coli from fatty acids to renewable fuels and chemicals such as ethanol, acetate, acetone, butanol, and propionate has previously been proposed (Dellomonaco et al. 2010), suggesting that fatty acids could become an effective carbon source for industrial production.
E. coli is used to produce several industrial primary metabolites, amino acids, and organic acids (Leuchtenberger et al. 2005; Wendisch et al. 2006). Among these, l-lysine is used in food and feed additives and is produced worldwide at quantities of over 1,500,000 metric tons per year. The use of this bacterium has economic advantages because of its fast growth and substrate consumption rates. In addition, more biochemical, molecular biological, and post-genomic data are available for these model organisms than for most others.
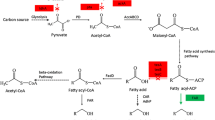
Fatty acids are assimilated and degraded to acetyl-CoA in E. coli by the β-oxidation-pathway proteins FadL, FadD, FadE, FadB, and FadA under both aerobic and anaerobic conditions (Cronan and Subrahmanyam 1998). All of the E. coli fatty acid β-oxidation pathway genes (fadL, fadD, fadE, fadB, fadA, fadI, and fadJ) and short-chain fatty acid utilizing genes (atoD, atoA, and atoB) have been identified (Jenkins and Nunn 1987), and most β-oxidation pathway genes are regulated by FadR (Cronan and Subrahmanyam 1998). FadR also upregulates fabA, fabB, and iclR genes and downregulates fad genes (fadL, fadD, fadE, fadB, fadA, fadI, and fadJ) and the uspA gene. fabA and fabB are involved in fatty acid synthesis (Magnuson et al. 1993), whereas IclR regulates acetyl-CoA metabolism through aceBA, which encodes glyoxylate shunt-pathway enzymes (Resnik et al. 1996). The uspA gene is induced by various stresses, including heat and oxidative (Nachin et al. 2005), and fabA overexpression decreases the monounsaturated fatty acid content of E. coli cell membranes, leading to increased cell resistance to oxidative stress or stress caused by reactive oxygen species (ROS)-generating compounds (Pradenas et al. 2012).
Here, we examined the mechanism of fatty acid degradation by E. coli to promote fatty acid utilization. The E. coli genome evolves and adapts to laboratory cultivation conditions (Fong et al. 2005; Herring et al. 2006). We therefore initiated wild-type E. coli cultivation for fatty acid utilization on minimal medium supplied with sodium oleate as the sole carbon source. Oleate was used because it is common in vegetable oils and is relatively easy to handle experimentally. We analyzed the physiological phenotype of the E. coli mutant obtained that could utilize oleate efficiently and investigated the effects of oxidative stress, especially those caused by ROS-generating compounds, on cell growth and lysine production.
Materials and methods
Bacterial strains and plasmids
All strains, plasmids, and primers used are listed in Table 1. The oxyS gene encoding an oxidative-stress regulator and its promoter region was amplified by the polymerase chain reaction (PCR) using the E. coli MG1655 genome and the primer set oxyS1 (5′-TACCCGGGGATCCTCTAGAGTTCCGCGAGGCGCACCATATTGTTGGTGAA-3′) and oxyS2 (5′-TTGCATGCCTGCAGGTCGACAGAAACGGAGCGGCACCTCTTTTAACCCT-3′).
The PCR product was purified with the Wizard SV gel and PCR clean-up system (Promega, Madison, WI), digested by SalI, and cloned into pTWV229 digested by SalI using the In-Fusion PCR cloning system (Clontech, Mountain View, CA). The resultant plasmid was designated pTWV228-oxyS.
The sodA gene encoding the superoxide dismutase overexpressing plasmid pTWV229-sodA was constructed as follows. The sodA open reading frame region was amplified using sodA1 (5′-TGATTACGCCAAGCTTAGGAGGTTAAATGAGCTATACCCTGCCATCCCTGCCGTA-3′) and sodA2 (5′- ATCCTCTAGAGTCGACGCGGCCGCTACTTATTTTTTCGCCGCAAAACGTGCCGCTGC-3′) primers. The PCR product was purified, digested by HindIII and SalI, and cloned into pTWV229 digested with the same restriction enzymes.
Adaptive evolution and analysis of an effective mutation
Minimal medium M9 (Miller 1992) supplemented with 1 mM MgSO4, 0.1 mM CaCl2, 0.001 % thiamine, 0.5 % Tween80 (polyoxyethylene sorbitan monooleate, CAS:9005-65-6), and 2 g/L sodium oleate was used in adaptive evolution experiments. One loop of E. coli MG1655 was inoculated onto an M9 plate and incubated for 20 h at 37 °C. Cells were cultured in L-shaped test tubes using a TN-2612 rocking incubator (Advantec, Tokyo, Japan) at 37 °C with constant shaking at 70 rpm. The optical density at 600 nm of the culture was measured continuously, and test-tube cultivation started at approximately OD600 0.006 and finished at OD600 0.3. The culture broth was transferred into fresh minimal medium, and the test-tube cultivation was repeated 22 times for a total cultivation time of 445 h. A single colony was then isolated from the resultant broth spread onto an M9 plate and designated FitnessOle.
The addition of Tween80 as an emulsifying agent of sodium oleate clarified the medium and allowed us to accurately measure the OD600 in fatty acid supplied medium (Suzuki et al., unpublished data). We ascertained that E. coli MG1655 and FitnessOle could not grow and utilize Tween80 as a sole carbon source in test-tube and flask cultivation using M9 medium (data not shown).
The FitnessOle genome was analyzed by whole genome sequencing with an Illumina Genome Analyzer II (GAII; Illumina Inc, San Diego, CA). In order to introduce the rpsA D210Y mutation into the genomes of other strains, a FitnessOle ycaI deletion mutant was constructed by PCR and the λ red deletion method using ycaI1 primer (5′-agacaaccgctcaacaaagttgcacactttccataaacagggaggggtgcTCTAGACGCTCAAGTTAGTATA-3′) and ycaI2 primer (5′-gttgtttgtagtgacgccagatactgtgcacgcaggctacaattcggttcAGATCTTGAAGCCTGCTTT-3′) as
ycaI is located close to rpsA in the genome. Because ycaI gene is located about 1.7 kbps of rpsA D210Y mutation in the E. coli genome and no significant phenotypes in this study were observed by ycaI gene deletion (data not shown), we can introduce the rpsA D210Y mutation with ycaI gene deletion by using the phage P1 without phenotypic influence. MG1655 containing the rpsA D210Y mutation and WC196LC containing the rpsA D210Y mutation were constructed by phage P1 transduction using the phage P1 obtained from the FitnessOle ycaI deletion strain.
Statistical testing and estimation of p values
The standard error of the mean calculation and a two-tailed unpaired Student’s t test were performed using Excel software (Microsoft Corporation, Redmond, WA) from more than three independent samples.
Culture conditions
For test-tube cultivation, E. coli MG1655 and its derivative strains were grown overnight at 37 °C on M9 plates supplemented with 1 mM MgSO4, 0.1 mM CaCl2, 0.001 % thiamine, and 2 g/L glucose. One loop of the grown cells was inoculated into 10 mL minimal medium M9 supplemented with 1 mM MgSO4, 0.1 mM CaCl2, 0.001 % thiamine, 0.5 % Tween80, and 1 g/L carbon source (sodium oleate or glucose) in L-shaped test tubes and cultivated at 37 °C with constant shaking at 70 rpm using a TN-2612 rocking incubator.
For flask cultivation, E. coli MG1655 and its derivative cells grown overnight at 37 °C on M9 plates supplemented with 1 mM MgSO4, 0.1 mM CaCl2, 0.001 % thiamine, and 2 g/L glucose were inoculated into 20 mL of M9 medium supplemented with 1 mM MgSO4, 0.1 mM CaCl2, 0.001 % thiamine, 0.5 % Tween80, and 10 g/L carbon source (oleic acid, elaidic acid, acetate, maltose, glycerol, or glucose) in a Sakaguchi flask (500 mL) at an initial OD600 of 0.2 and cultivated at 37 °C with reciprocal shaking at 120 rpm. The pH of each component was adjusted to 7.0 before sterilization.
For l-lysine fermentation from fatty acids in flasks, E. coli strains derived from WC196LC (Leuchtenberger et al. 2005) were cultivated overnight at 37 °C on LB plates composed of 1.0 % Bacto tryptone, 0.5 % Bacto yeast extract, 1 % NaCl, and 1.5 % agar. Cells were then inoculated into 40 mL flask-fermentation medium comprising 2 g/L yeast extract, 1 g/L MgSO4·7H2O, 24 g/L (NH4)2SO4, 1 g/L KH2PO4, 0.01 g/L FeSO4·7H2O, 0.082 g/L MnSO4·7H2O, 20 g/L PIPES, and 10 g /L sodium oleate in Erlenmeyer flasks (500 mL) at an initial OD600 of 0.25. The pH of the medium was adjusted to 7.0 before sterilization. Fermentation was performed at 37 °C with rotary shaking at 200 rpm.
For l-lysine fermentation from fatty acids in a jar fermenter, E. coli WC196LC and its derivative strains grown overnight at 37 °C on LB plates were transferred to 300 mL jar-fermentation medium comprising 2 g/L yeast extract, 1 g/L MgSO4·7H2O, 24 g/L (NH4)2SO4, 1 g/L KH2PO4, 0.01 g/L FeSO4·7H2O, 0.082 g/L MnSO4·7H2O, and 10 g/L carbon source (sodium oleate or glucose) in 1-L glass vessels (Able Corporation, Tokyo, Japan) at an initial OD600 of 0.04 and subjected to batch cultivation in jar fermenters DPC-2A (Able Corporation, Tokyo, Japan) at 37 °C. The pH of the culture was maintained at 6.7 by adding ammonia gas.
Analytical methods
Aggregation indexes of E. coli MG1655 and the FitnessOle strain were measured as previously described (Malik et al. 2003). Cell growth was analyzed by measuring the OD600 with a spectrophotometer U-2900 (Hitachi, Tokyo, Japan) and by counting the number of living cells. Tween80 solution (10 %) was used for dilution to eliminate the influence of fatty acids on OD600nm. Living cell counting in the fermentation broth was carried out by diluting the broth with saline and counting the number of colonies on LB plates after cultivation for 24 h at 37 °C. The maximum specific growth rate (μ max) and maximum specific substrate-consumption rate (ν max) were calculated by nine-dimension polynomial approximations using the numerical computation software package MATLAB (MathWorks, Natick, MA). R-squared values of the approximations were greater than 0.995.
Carbonylated protein concentrations were measured using a protein carbonyl colorimetric assay kit (Cayman Chemical Company, Ann Arbor, MI). To measure cells in the same growth phase, we sampled the cells from the flask-fermentation broth when the residual carbon source concentration reached 1 g/L for the carbonylated protein assay and measured the OD600 to confirm that sampled cells were divided a similar number of times. Glucose and l-lysine were assayed by a biotech analyzer AS310 (Sakura Si Co., Ltd., Tokyo, Japan). Glycerol was assayed by an electrochemical biosensor BF-5 (Oji Scientific Instruments, Hyogo, Japan), maltose by an ion chromatography system ICS-3000 (Dionex, Sunnyvale, CA), acetate by a liquid chromatograph LC-10AD (Shimadzu, Kyoto, Japan), and oleic acid and elaidic acid by a gas chromatograph GC-2014 (Shimadzu).
Intracellular hydrogen peroxide (H2O2) was measured as previously described (González-Flecha and Demple 1999; Maisonneuve et al. 2008). Briefly, bacterial cells were collected from culture broths by centrifugation (13,800×g) for 2 min at 4 °C and resupended in phosphate buffer (pH 7.3) at an approximate density of 106 cells/mL. After 10 min diffusion of intracellular H2O2 into the buffer through cellular membranes, the cells were removed by centrifugation at 13,800×g for 2 min at 4 °C. Then, 10 μL supernatant was suspended separately in solution A (2 μM horseradish peroxidase and 10 μM HPF (Maisonneuve et al. 2008) in 100 mM phosphate buffer (pH 7.3)) and solution B (2 μM catalase and 10 μM HPF (Maisonneuve et al. 2008) in 100 mM phosphate buffer solution (pH 7.3)).
The resusupended samples were incubated at 37 °C for 75 min in the dark, and the emitted fluorescence at 515 nm was measured using excitation at 490 nm. The intracellular H2O2 concentration was calculated by subtracting the fluorescence of solution B from that of solution A.
Results
Acquisition of fatty acid-utilizing E. coli mutant strain by laboratory evolution and analysis of physiological phenotypes
To improve fatty acid utilization by E. coli, we attempted to obtain a mutant with enhanced function. We cultivated the wild-type E. coli strain MG1655 in minimal media supplemented with sodium oleate as a sole carbon source for 445 h. We then isolated a mutant with improved utilization of fatty acid and designated it FitnessOle. Figure 1 shows the growth (Fig. 1a) and oleate concentration (Fig. 1b) profiles of the wild-type and FitnessOle strains in flask culture. The FitnessOle strain showed significantly enhanced growth in oleate culture with enhanced consumption of oleate. We ascertained that four other independent colonies isolated from the same broth after 445 h cultivation showed the same enhanced growth phenotype as the FitnessOle strain in oleate culture (data not shown). The FitnessOle strain also showed higher μ max and ν max values when grown on fatty acids (oleic or elaidic acids), glycerol, or acetate as the sole carbon source compared with the wild-type strain (Table 2). The FitnessOle strain also showed increased cell biomass accumulation when grown on oleic acid as the sole carbon source under aerobic conditions compared with the wild-type strain. But the FitnessOle strain showed the same cell biomass accumulation as the wild-type strain when grown on oleic acid under anaerobic conditions (data not shown). Cell aggregation of the FitnessOle strain appeared to be facilitated compared with the wild-type strain. We also measured the aggregation index (Malik et al. 2003) and found the aggregation tendency of the FitnessOle strain to be significantly increased (Fig. 2c).
The physiological phenotypes of the FitnessOle strain cell growth (a) and residual oleate concentration (b) profiles were measured for MG1655 (solid circle) and FitnessOle strains (solid triangle). Carbonylated protein content (c) and intracellular hydrogen peroxide concentration (d) were also measured. Values are the mean of more than three independent samples. SE bars represent the standard error of the mean calculated with Excel software
DNA and amino acid sequence of rpsA D210Y mutation (a) and its effect on fatty acid utilization. b Growth of the rpsA D210Y mutant strain in M9 medium test tube cultivation supplemented with sodium oleate as the sole carbon source. Parental strain MG1655 (solid circle), FitnessOle strain (solid triangle), MG1655 ΔycaI::attR-cat-attL strain (solid square) and MG1655 rpsA D210Y ΔycaI::attR-cat-attL strain (empty diamond)were monitored. Aggregation index (c) and intracellular hydrogen peroxide concentration (d) of cells cultivated in M9 medium supplemented with sodium oleate as a sole carbon source were also measured. Values are the mean of more than three independent samples. SE bars represent the standard error of the mean calculated with Excel software
The uspA gene, encoding a universal stress protein with unknown functions, has been reported to be a target of FadR that is upregulated when E. coli is exposed to oxidative stress (Nachin et al. 2005). FadR functions as switch between fatty acid β-oxidation and fatty acid biosynthesis (Xu et al. 2001). Based on these facts, we investigated the relationship between fatty acid utilization and oxidative stress. We measured the carbonylated protein concentration, a major oxidative-stress marker, of E. coli cells utilizing glucose or oleate as the sole carbon source (Maisonneuve et al. 2008) and found it to be decreased in the FitnessOle strain compared with wild type after a similar number of cell divisions (Fig. 1c), indicating decreased oxidative stress in this strain. We next measured the concentration of intracellular H2O2, a major ROS, and also found it to be decreased in the FitnessOle strain compared with wild type when the cells utilized oleate as the sole carbon source (Fig. 1d).
To identify an effective mutation for fatty acid utilization in the genome of the fatty acid-utilizing E. coli mutant strain, we carried out whole genome sequencing and discovered the rpsA D210Y mutation (Fig. 2a). The rpsA D210Y mutation was present in the genomes of four other independent colonies isolated from the broth after 445 h minimal media cultivation. Introduction of this mutation into the MG1655 genome resulted in enhanced cell growth when oleate was used as the sole carbon source (Fig. 2b) and higher μ max and ν max values than those of the wild-type MG1655. Furthermore, introduction of the rpsA D210Y mutation caused a decrease in the concentration of intracellular H2O2 when the strain utilized oleate as a sole carbon source (Fig. 2d) despite no significant change in aggregation index (Fig. 2c). Introduction of the rpsA D210Y mutation into the genome of the E. coli L-lysine producer strain WC196LC/pCABD2 (Kikuchi et al. 1997) resulted in increased cell growth and l-lysine accumulation (Table 3). There were, however, no apparent differences in l-lysine production, cell growth, or glucose consumption following introduction of the rpsA D210Y mutation when the E. coli l-lysine producer strain utilized glucose (Table 3).
Promotion of fatty acid utilization by reducing intracellular H2O2
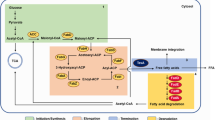
In the wild-type strain MG1655, no carbon source (glucose or oleate)-dependent changes in carbonylated protein accumulation were observed (Fig. 1c), suggesting that the specific ROS stress was decreased only in the fatty acid-utilizing mutant FitnessOle strain. As shown in Fig. 3, the ROS consists of a single oxygen molecule, a superoxide anion (O2∙−), H2O2, and a hydroxyl radical (∙OH) (González-Flecha and Demple 1999; Blanchard et al. 2007; Zheng et al. 1998). The two transcription factors reported to respond to O2∙− and H2O2 are also shown (Blanchard et al. 2007; Zheng et al. 1998). SoxR is mainly involved in defense against O2∙− and OxyR mainly against H2O2. Thus, we investigated the growth of the soxR deletion mutant (JW3933) and the oxyR deletion mutant (JW4024) derived from BW25113 (Baba et al. 2006) on glucose or oleate as the sole carbon source. Both the ΔsoxR and ΔoxyR strains showed no significant stationary phase optical density changes compared with their host strain BW25113 when they utilized glucose as the sole carbon source (Fig. 4a). However, the ΔoxyR strain showed an apparent cell growth defect and a stationary phase optical density decrease when grown on sodium oleate as the sole carbon source (Fig. 4b).
Schematic representation of ROS generation and elimination, and transcriptional regulation of ROS elimination systems in E. coli. Ordinary electron transfer to an oxygen molecule converting into a water molecule is catalyzed by cytochrome oxidases, but incomplete electron transfer to an oxygen molecule generates O2 ⋅−, H2O2, and OH−. These ROS molecules cause cell damage so E. coli possesses various ROS scavenger genes. Superoxide dismutases (SodA and SodB) convert O2 ⋅− into H2O2, which decomposes into harmless H2O and O2 with the aid of catalases KatG, KatE, and the alkyl hydroxiperoxide reductase AhpCF. Intracellular H2O2 excretion is promoted by small RNA oxyS. These ROS-scavenger genes are regulated by SoxR and OxyR. SoxR detects intracellular O2 ⋅− and upregulates SoxS expression. SoxR and SoxS activate O2 ⋅− decomposing genes such as sodA and sodB. OxyR is an intracellular H2O2 sensor and H2O2 removal-associated gene regulator. OxyR activates oxyS and H2O2-decomposing genes such as katG, katE, and ahpCF
The effects of antioxidant transcription factor gene deletion. The growth of ΔsoxR and ΔoxyR strains in M9 medium test tube cultivation supplemented with sodium glucose (a) or oleate (b) as the sole carbon source. Parental strain BW25113 (solid circle), ΔoxyR strain (solid triangle), and ΔsoxR strain (solid square) were monitored. Values are the mean of more than three independent samples. SE bars represent the standard error of the mean calculated with Excel software
To determine which ROS has the greatest negative effect on fatty acid utilization in E. coli, we constructed expression plasmids harboring the sodA gene encoding the O2∙– scavenger dismutase or the oxyS gene encoding an oxidative-stress regulator and introduced them into the wild-type strain MG1655. The oxyS transcript might be involved in the excretion, rather than removal, of H2O2 by catalase peroxidases (González-Flecha and Demple 1999). The resultant strains (MG1655/pTWV229-sodA and MG1655/pTWV228-oxyS) were cultivated on glucose or sodium oleate as a carbon source (Fig. 5). The intracellular H2O2 concentration was significantly increased when the E. coli MG1655/pTWV228 strain utilized sodium oleate compared with glucose as the sole carbon source (Fig. 5a, b; p < 0.03, Student’s t test). Furthermore, overexpression of the sodA gene resulted in an increase of intracellular H2O2 and a severe growth defect (Fig. 5b, d). Overexpression of the oxyS gene decreased intracellular H2O2 levels and promoted cell growth (Fig. 5b, d).
Effects of overexpression of sodA or oxyS genes. Intracellular hydrogen peroxide concentration of cells grown in M9 medium supplemented with glucose (a) or sodium oleate (b) as the sole carbon source. Cell growth using glucose (c) or sodium oleate (d) as the sole carbon source. Vector control MG1655/pTWV228 (solid circle), oxyS overexpressing strain MG1655/pTWV228-oxyS (solid triangle) and sodA overexpressing strain MG1655/pTWV229-sodA (solid square) were studied. Values are the mean of more than three independent samples. SE bars represent the standard error of the mean calculated with Excel software. The Student’s t test between the intracellular hydrogen peroxide concentration of vector control samples and that of oxyS overexpression samples when grown on sodium oleate gave a p value of 0.024
Effects of promotion of fatty acid utilization by reducing ROS stress on l-lysine production
To investigate the relationship between material production from fatty acids and reduction of ROS stress, we used the E. coli l-lysine-producing strain WC196LC/pCABD2 (Kikuchi et al. 1997). Overexpression of oxyS in WC196LC/pCABD2 resulted in increased cell growth and l-lysine accumulation (Table 4). This suggests that decreased ROS stress, assumed to be mainly caused by intracellular H2O2, promoted fatty acid utilization and l-lysine production. Next, we investigated the effect of the antioxidant reagent thiourea on fatty acid utilization in larger scale fermentation. Thiourea reduces damage caused by H2O2 (Blount et al. 1989) and was shown to decrease intracellular H2O2 concentrations both in glucose and sodium oleate utilization (Table 5). However, thiourea also reduced the cultivation times required to consume fatty acid and increased cell and l-lysine accumulation when the E. coli l-lysine-producing strain utilized sodium oleate (Table 5). No apparent differences in l-lysine production, cell growth, or glucose consumption were observed following thiourea addition when the E. coli l-lysine producer strain utilized glucose (Table 5).
Discussion
We predicted that test-tube adaptive evolution would shed light on fatty acid utilization in E. coli by comparing physiological phenotype differences between a mutant that can utilize fatty acids efficiently and a wild-type strain. Indeed, the mutant strain FitnessOle showed a higher ability to utilize various fatty acids, including oleic acid, elaidic acid (Table 2), stearic acid, sodium palmitate, myristic acid, and sodium oleate (data not shown), compared with wild type.
The FitnessOle strain showed enhanced cell aggregation as well as lowered ROS stress (Fig. 2). Microorganism aggregation can be quantified by measurement of the aggregation index and is positively correlated with membrane hydrophobicity (Malik et al. 2003). Thus, our results indicate that the FitnessOle strain possesses increased membrane hydrophobicity; we will examine this together with its relationship with fatty acid utilization in a future study.
Our main focus here was the promotion of fatty acid utilization in E. coli. We discovered a novel fatty acid utilization promoting mutation, rpsA D210Y. rpsA encodes the 30S ribosomal subunit protein. Introduction of the rpsA D210Y mutation decreased intracellular H2O2 concentrations (Fig. 2d) but had no effect on cell aggregation (Fig. 2c). These results suggest that intracellular H2O2 concentrations can influence fatty acid utilization. We also found that a decrease in ROS stress, particularly that of H2O2, was important to enhance the ability to utilize fatty acid in E. coli. However, as the ROS was shown to change (Fig. 3), it is difficult to identify which species affects fatty acid utilization. Nevertheless, our research revealed that it was mainly inhibited by H2O2 rather than O2∙− (Figs. 4 and 5). There were no apparent changes for the carbonylated protein accumulation in wild-type E. coli cells grown in M9 media with glucose or oleate (Fig. 1c). On the other hand, the intracellular H2O2 concentration was significantly increased when the wild-type strain utilized sodium oleate compared with glucose as the sole carbon source (Fig. 5a, b). Carbonylated protein content indicates total ROS stress including , H2O2, O2∙–, and ∙OH (Fig. 3). These data show that the specific ROS stress is H2O2 stress when E. coli utilized sodium oleate among the various ROS. Our preliminary microarray research revealed that katG, ahpC, ahpF, and oxyR transcripts were increased in FitnessOle strain compared with the wild type when they utilized sodium oleate (Doi et al., unpublished data). In addition, our preliminary microarray research also revealed that sodA, sodB, soxS, and soxR transcripts were decreased in FitnessOle strain compared with the wild type when they utilized sodium oleate (Doi et al., unpublished data). These results indicate the independence of H2O2 and O2∙–. No significant phenotype was previously reported following the overexpression of oxyS when E. coli was cultivated on LB, in which the main carbon source was amino acids not fatty acids (González-Flecha and Demple 1999).
Similarly, no significant phenotype was observed by the overexpression of oxyS when E. coli utilized glucose as the main carbon source (Fig. 5a, c). However, we did observe the promotion of fatty acid utilization following oxyS overexpression, presumably because of reduced H2O2 levels (Fig. 5b and d) and l-lysine production (Table 4). We assumed that this effect was a result of H2O2 excretion by the oxyS transcript (González-Flecha and Demple 1999). These results demonstrate a specific phenotype after oxyS overexpression and suggest that more H2O2 is generated when utilizing fatty acid compared with glucose and amino acids supplied in LB medium. We presume that H2O2 is generated by flavin adenine dinucleotide (FADH2) during the fatty acid β-oxidation pathway.
This autoxidation of FADH2 is a well-known phenomenon that occurs, for example, in glucose concentration analysis by glucose oxidase (Raba and Mottola 1995). Free FADH2 was previously shown to be reduced by cytosolic enzymes such as l-aspartate oxidase and was autoxidized to generate endogenous E. coli H2O2 (Korshunov and Imlay 2010; Messner and Imlay 2002). We assumed that FADH2 reduced by FadE, the acyl-CoA dehydrogenase in the fatty acid β-oxidation pathway, would be the endogenous H2O2 source in the present study. We are currently investigating the effects of FADH2 oxidation by electron-transfer-flavoprotein (ETF) or ETF dehydrogenase (EC 1.5.5.1). Our preliminary research revealed that the overexpression of these homologous genes in E. coli results in the decrease of intracellular H2O2 and increased l-lysine accumulation during the utilization of fatty acid as a carbon source (Hoshino et al., unpublished data), supporting our hypothesis of endogenous H2O2 generation by FADH2 autoxidation.
In jar fermentation, thiourea addition decreased intracellular H2O2 concentrations, lowered cultivation time, and increased l-lysine production when E. coli utilized fatty acid (Table 5). However, when glucose was utilized, the total cultivation time and l-lysine production remained the same, even though intracellular H2O2 concentrations decreased (Table 5). This suggests that higher ROS stress was generated following fatty acid utilization, which inhibited growth. The addition of thiourea, a common antioxidant molecule, to reduce ROS stress is a promising approach for fatty acids used on an industrial scale as raw materials for fermentation. Thiourea is used as a building material because it is inexpensive compared with antioxidants such as vitamin C or tocopherol.
The present study focused only on the physiological phenotype of the FitnessOle strain and identified a novel mutation, rpsA D210Y. We are currently using this mutation to understand the mechanism of decreasing intracellular H2O2 concentrations. Our preliminary data revealed that superoxide dismutase SodB protein expression decreased following the introduction of the rpsA D210Y mutation, as shown by 2-D electrophoresis and liquid chromatography–mass spectrometry analysis (Doi et al., unpublished data). However, the rpsA D210Y mutation alone could not achieve the increased cell growth and decreased intracellular H2O2 concentration shown by the FitnessOle strain. Therefore, we are now investigating the other mutations of the FitnessOle genome, which should reveal more information concerning E. coli fatty acid utilization. We expect this to clarify the relationship between the reduction of ROS and changes in membrane hydrophobicity.
Fatty acids are a promising raw material for substance production, and we have shown that they can be used as such for amino acid fermentation by means of emulsification, despite their insolubility in water (Suzuki et al., unpublished data). This report is the first to show the bioconversion of fatty acid into L-lysine by obtaining a fatty acid-utilizing mutant, the FitnessOle strain. H2O2 generated by fatty acid β-oxidation was revealed to have a critical effect on growth and lysine production when E. coli utilized fatty acid as a carbon source. This will be useful for future industrial production using fatty acids as substrates, and we hope to identify further useful insights to help in the realization of this process.
References
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008
Blanchard JL, Wholey WY, Conlon EM, Pomposiello PJ (2007) Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks. PLoS ONE 11:e1186
Blount S, Griffiths HR, Lunec J (1989) Reactive oxygen species induce antigenic changes in DNA. FEBS Lett 245(1):100–104
Chen CY, Yeh KL, Aisyah R, Lee DJ, Chang JS (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102(1):71–81
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Cronan JE Jr, Subrahmanyam S (1998) FadR, transcriptional co-ordination of metabolic expediency. Mol Microbiol 29(4):937–943
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645
Dellomonaco C, Rivera C, Campbell P, Gonzalez R (2010) Engineered respiro-fermentative metabolism for the production of biofuels and biochemicals from fatty acid-rich feedstocks. Appl Environ Microbiol 76(15):5067–5078
Fong SS, Joyce AR, Palsson BO (2005) Parallel adaptive evolution cultures of Escherichia coli lead to convergent growth phenotypes with different gene expression states. Genome Res 15(10):1365–1372
González-Flecha B, Demple B (1999) Role for the oxyS gene in regulation of intracellular hydrogen peroxide in Escherichia coli. J Bacteriol 181(12):3833–3836
Herring CD, Raghunathan A, Honisch C, Patel T, Applebee MK, Joyce AR, Albert TJ, Blattner FR, van den Boom D, Cantor CR, Palsson BO (2006) Comparative genome sequencing of Escherichia coli allows observation of bacterial evolution on a laboratory timescale. Nat Genet 38(12):1406–1412
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Jenkins LS, Nunn WD (1987) Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J Bacteriol 169(1):42–52
Katashkina JI, Skorokhodova AY, Zimenkov DV, Gulevich AY, Minaeva NI, Doroshenko VG, Biryukova IV, Mashko SV (2005) Tuning the expression level of a gene located on a bacterial chromosome. Mol Biol 39(5):719–726
Kikuchi Y, Kojima H, Tanaka T, Takatsuka Y, Kamio Y (1997) Characterization of second lysine decarboxylase isolated from Escherichia coli. J Bacteriol 179(14):4486–4492
Kojima H, Ogawa Y, Kawamura K, Sano K (1994) Treaty patent WO95/16042. International Patent Cooperation
Korshunov S, Imlay JA (2010) Two sources of endogenous hydrogen peroxide in Escherichia coli. Mol Microbiol 75(6):1389–1401
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69(1):1–8
Magnuson K, Jackowski S, Rock CO, Cronan JE Jr (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57(3):522–542
Maisonneuve E, Fraysse L, Lignon S, Capron L, Dukan S (2008) Carbonylated proteins are detectable only in a degradation-resistant aggregate state in Escherichia coli. J Bacteriol 190(20):6609–6614
Malik A, Sakamoto M, Hanazaki S, Osawa M, Suzuki T, Tochigi M, Kakii K (2003) Coaggregation among nonflocculating bacteria isolated from activated sludge. Appl Environ Microbiol 69(10):6056–6063
Messner KR, Imlay JA (2002) Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J Biol Chem 277(45):42563–42571
Miller JH (1992) A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Nachin L, Nannmark U, Nyström T (2005) Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J Bacteriol 187(18):6265–6272
Pradenas GA, Paillavil BA, Reyes-Cerpa S, Pérez-Donoso JM, Vásquez CC (2012) Reduction of the monounsaturated fatty acid content of Escherichia coli results in increased resistance to oxidative damage. Microbiology 158(5):1279–1283
Raba J, Mottola HA (1995) Glucose oxidase as an analytical reagent. Crit Rev Anal Chem 25(1):1–42
Resnik E, Pan B, Ramani N, Freundlich M, LaPorte DC (1996) Integration host factor amplifies the induction of the aceBAK operon of Escherichia coli by relieving IclR repression. J Bacteriol 178(9):2715–2717
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19(5):430–436
Service RF (2009) ExxonMobil fuels Venter’s efforts to run vehicles on algae-based oil. Science 325(5939):379
Wendisch VF, Bott M, Eikmanns BJ (2006) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr Opin Microbiol 9(3):268–274
Xu Y, Heath RJ, Li Z, Rock CO, White SW (2001) The FadR.DNA complex. Transcriptional control of fatty acid metabolism in Escherichia coli. J Biol Chem 276(20):17373–17379
Zheng M, Åslund F, Storz G (1998) Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 179(5357):1718–1721
Acknowledgments
The authors thank S. Suzuki, H. Kobayashi, and M. Sada for providing unpublished information about cultivation methods using fatty acids as carbon sources. We are also grateful to H. Motokawa and S. Fukai for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Doi, H., Hoshino, Y., Nakase, K. et al. Reduction of hydrogen peroxide stress derived from fatty acid beta-oxidation improves fatty acid utilization in Escherichia coli . Appl Microbiol Biotechnol 98, 629–639 (2014). https://doi.org/10.1007/s00253-013-5327-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5327-6