Abstract
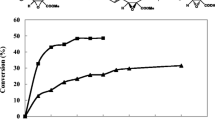
Efficient and highly enantioselective hydrolysis of 2-carboxyethyl-3-cyano-5-methylhexanoic acid ethyl ester (CNDE) is the most crucial step in chemoenzymatic synthesis of Pregabalin. By using site-saturation mutagenesis and high-throughput screening techniques, lipase Lip from Thermomyces lanuginosus DSM 10635 was engineered to improve its activity towards CNDE. The triple mutant, S88T/A99N/V116D exhibited a 60-fold improvement in specific activity for CNDE (2.35 U/mg) over the wild-type Lip (0.039 U/mg). Modeling and docking studies demonstrated that the mutant could more effectively stabilize oxygen anions in transition states and the lid of Lip in the open conformation. Additionally, the kinetic resolution of CNDE catalyzed by Escherichia coli cell overexpressing S88T/A99N/V116D mutant afforded (3S)-2-carboxyethyl-3-cyano-5-methylhexanoic acid in 42.4 % conversion and 98 % ee within 20 h with a substrate loading of 1 M (255 g/l). These results demonstrated that a novel and promising biocatalyst was created for efficient chemoenzymatic manufacturing of Pregabalin.






Similar content being viewed by others
References
Berg OG, Cajal Y, Butterfoss GL, Grey RL, Alsina MA, Yu BZ, Jain MK (1998) Interfacial activation of triglyceride lipase from Thermomyces (Humicola) lanuginosa: kinetic parameters and a basis for control of the lid. Biochemistry 37:6615–6627
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–252
Brzozowski AM, Savage H, Verma CS, Turkenburg JP, Lawson DM, Svendsen A, Patkar S (2000) Structural origins of the interfacial activation in Thermomyces (Humicola) lanuginosa lipase. Biochemistry 39:15071–15082
Cambon E, Piamtongkam R, Bordes F, Duquesne S, Andre I, Marty A (2010) Rationally engineered double substituted variants of Yarrowia lipolytica lipase with enhanced activity coupled with highly inverted enantioselectivity towards 2-bromo phenyl acetic acid esters. Biotechnol Bioeng 106:852–859
Carriere F, Thirstrup K, Hjorth S, Ferrato F, Nielsen PF, WithersMartinez C, Cambillau C, Boel E, Thim L, Verger R (1997) Pancreatic lipase structure–function relationships by domain exchange. Biochemistry 36:239–248
Chronopoulou EG, Labrou NE (2011) Site-saturation Mutagenesis: a powerful tool for structure-based design of combinatorial mutation libraries. Dunn B (ed) Current protocols in protein science. John Wiley, New York, 63: 26.6.1–26.6.10
Dalby PA (2011) Strategy and success for the directed evolution of enzymes. Curr Opin Struc Biol 21:473–480
de Carvalho CCCR (2011) Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv 29:75–83
De Groeve MRM, Remmery L, Van Hoorebeke A, Stout J, Desmet T, Savvides SN, Soetaert W (2010) Construction of cellobiose phosphorylase variants with broadened acceptor specificity towards anomerically substituted glucosides. Biotechnol Bioeng 107:413–420
Felluga F, Pitacco G, Valentin E, Venneri CD (2008) A facile chemoenzymatic approach to chiral non-racemic beta-alkyl-gamma-amino acids and 2-alkylsuccinic acids. A concise synthesis of (S)-(+)-Pregabalin. Tetrahedron-Asymmetr 19:945–955
Fernandez-Lafuente R (2010) Lipase from Thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. J Mol Catal B-Enzym 62:197–212
Garcia-Galan C, Berenguer-Murcia A, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of different enzyme immobilization strategies to improve enzyme performance. Adv Syn Catal 353(16):2885–2904
Gerstenbruch S, Wulf H, Mussmann N, O'Connell T, Maurer KH, Bornscheuer UT (2012) Asymmetric synthesis of d-glyceric acid by an alditol oxidase and directed evolution for enhanced oxidative activity towards glycerol. Appl Microbiol Biotechnol 96:1243–1252
Hernandez K, Fernandez-Lafuente R (2011) Control of protein immobilization: coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb Technol 48(2):107–122
Hoelsch K, Suhrer I, Heusel M, Weuster-Botz D (2013) Engineering of formate dehydrogenase: synergistic effect of mutations affecting cofactor specificity and chemical stability. Appl Microbiol Biotechnol 97:2473–2481
Holzwarth HC, Pleiss J, Schmid RD (1997) Computer-aided modelling of stereoselective triglyceride hydrolysis catalyzed by Rhizopus oryzae lipase. J Mol Catal B-Enzym 3:73–82
Hu SH, Martinez CA, Tao JH, Tully WE, Patrick K, Dumond Y (2011) Preparation of Pregabalin and related compounds. US8134023B2.
Lambertus T (2009) Processes for making Pregabalin and intermediates therefor. WO2009149928
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck — a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291
Li ZW, Roccatano D, Lorenz M, Schwaneberg U (2012) Directed evolution of subtilisin E into a highly active and guanidinium chloride- and sodium dodecylsulfate-tolerant protease. ChemBioChem 13:691–699
Martinez CA, Hu SH, Dumond Y, Tao JH, Kelleher P, Tully L (2008) Development of a chemoenzymatic manufacturing process for pregabalin. Org Process Res Dev 12:392–398
Martinez R, Jakob F, Tu R, Siegert P, Maurer KH, Schwaneberg U (2013) Increasing activity and thermal resistance of Bacillus gibsonii alkaline protease (BgAP) by directed evolution. Biotechnol Bioeng 110:711–720
Mordukhova EA, Lee HS, Pan JG (2008) Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl Environ Microbiol 74:7660–7668
Moris-Varas F, Shah A, Aikens J, Nadkarni NP, Rozzell JD, Demirjian DC (1999) Visualization of enzyme-catalyzed reactions using pH indicators: rapid screening of hydrolase libraries and estimation of the enantioselectivity. Bioorg Med Chem 7:2183–2188
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Norin M, Haeffner F, Achour A, Norin T, Hult K (1994) Computer modeling of substrate-binding to lipases from Rhizomucor miehei, Humicola lanuginosa, and Candida rugosa. Protein Sci 3:1493–1503
Peters GH, Svendsen A, Langberg H, Vind J, Patkar SA, Toxvaerd S, Kinnunen PKJ (1998) Active serine involved in the stabilization of the active site loop in the Humicola lanuginosa lipase. Biochemistry 37:12375–12383
Pleiss J, Fischer M, Peiker M, Thiele C, Schmid RD (2000) Lipase engineering database — understanding and exploiting sequence–structure–function relationships. J Mol Catal B-Enzym 10:491–508
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Rakels JLL, Straathof AJJ, Heijnen JJ (1993) A simple method to determine the enantiomeric ratio in enantioselective biocatalysis. Enzyme Microb Technol 15:1051–1056
Reetz MT (2012) Laboratory evolution of stereoselective enzymes as a means to expand the toolbox of organic chemists. Tetrahedron 68:7530–7548
Reetz MT, Wilensek S, Zha DX, Jaeger KE (2001) Directed evolution of an enantioselective enzyme through combinatorial multiple-cassette mutagenesis. Angew Chem Int Edit 40:3589–3591
Reetz MT, Prasad S, Carballeira JD, Gumulya Y, Bocola M (2010) Iterative saturation mutagenesis accelerates laboratory evolution of enzyme stereoselectivity: rigorous comparison with traditional methods. J Am Chem Soc 132:9144–9152
Romdhane IB, Frikha F, Maalej-Achouri I, Gargouri A, Belghith H (2012) Gene cloning and molecular characterization of the Talaromyces thermophilus lipase catalyzed efficient hydrolysis and synthesis of esters. Gene 494:112–118
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New York
Sanchis J, Fernandez L, Carballeira J, Drone J, Gumulya Y, Hobenreich H, Kahakeaw D, Kille S, Lohmer R, Peyralans J, Podtetenieff J, Prasad S, Soni P, Taglieber A, Wu S, Zilly F, Reetz M (2008) Improved PCR method for the creation of saturation mutagenesis libraries in directed evolution: application to difficult-to-amplify templates. Appl Microbiol Biotechnol 81:387–397
Schmid RD, Verger R (1998) Lipases: interfacial enzymes with attractive applications. Angew Chem Int Edit 37:1609–1633
Shu ZY, Duan MJ, Yang JK, Xu L, Yan YJ (2009) Aspergillus niger lipase: heterologous expression in Pichia pastoris, molecular modeling prediction and the importance of the hinge domains at both sides of the lid domain to interfacial activation. Biotechnol Prog 25:409–416
Silverman RB (2008) From basic science to blockbuster drug: the discovery of Lyrica. Angew Chem Int Edit 47:3500–3504
Simon L, Goodman JM (2010) Enzyme catalysis by hydrogen bonds: the balance between transition state binding and substrate binding in oxyanion holes. J Org Chem 75:1831–1840
Turner NJ (2009) Directed evolution drives the next generation of biocatalysts. Nat Chem Biol 5:568–574
Verger R (1997) ‘Interfacial activation’ of lipases: facts and artifacts. Nat Chem Biol 15:32–38
Wang J, Wang D, Wang B, Mei ZH, Liu J, Yu HW (2012a) Enhanced activity of Rhizomucor miehei lipase by directed evolution with simultaneous evolution of the propeptide. Appl Microbiol Biotechnol 96:443–450
Wang M, Si T, Zhao HM (2012b) Biocatalyst development by directed evolution. Bioresour Technol 115:117–125
Wenda S, Illner S, Mell A, Kragl U (2011) Industrial biotechnology—the future of green chemistry? Green Chem 13:3007–3047
Woodley JM (2008) New opportunities for biocatalysis: making pharmaceutical processes greener. Trends Biotechnol 26:321–327
Xie ZY, Feng JL, Garcia E, Bernett M, Yazbeck D, Tao JH (2006) Cloning and optimization of a nitrilase for the synthesis of (3S)-3-cyano-5-methyl hexanoic acid. J Mol Catal B-Enzym 41:75–80
Yapoudjian S, Ivanova MG, Brzozowski AM, Patkar SA, Vind J, Svendsen A, Verger R (2002) Binding of Thermomyces (Humicola) lanuginosa lipase to the mixed micelles of cis-parinaric acid/NaTDC — fluorescence resonance energy transfer and crystallographic study. Eur J Biochem 269:1613–1621
Zheng YY, Guo XH, Song NN, Li DC (2011) Thermophilic lipase from Thermomyces lanuginosus: gene cloning, expression and characterization. J Mol Catal B-Enzym 69:127–132
Zheng RC, Li AP, Wu ZM, Zheng YG (2012) Enzymatic production of (S)-3-cyano-5-methylhexanoic acid ethyl ester with high substrate loading by immobilized Pseudomonas cepacia lipase. Tetrahedron-Asymmetry 23:1517–1521
Zheng RC, Wang TZ, Fu DJ, Li AP, Li XJ, Zheng YG (2013) Biocatalytic synthesis of chiral intermediate of Pregabalin with high substrate loading by a newly isolated Morgarella morganii ZJB-09203. Appl Microbiol Biotechnol 97:4839–4847
Acknowledgements
This work was financially supported by the National High Technology Research and Development Program of China (no. 2012AA022201), the Key Scientific and Technology Programs of Zhejiang Province (no. 2012C03005-2) and the Natural Science Foundation of Zhejiang Province (no. Z4090612).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XJ., Zheng, RC., Ma, HY. et al. Engineering of Thermomyces lanuginosus lipase Lip: creation of novel biocatalyst for efficient biosynthesis of chiral intermediate of Pregabalin. Appl Microbiol Biotechnol 98, 2473–2483 (2014). https://doi.org/10.1007/s00253-013-5136-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5136-y