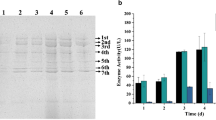
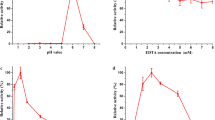
Abstract
Catalytic and physicochemical properties of representative fungal dye-decolorizing peroxidases (DyPs) of wood- (WRF) and litter-decomposing white-rot fungi (LDF) are summarized and compared, including one recombinant Mycetinis scorodonius DyP (rMscDyP; LDF), the wild-type Auricularia auricula-judae DyP (AauDyP; WRF), and two new DyPs secreted by the jelly fungi Exidia glandulosa (EglDyP; WRF) and Mycena epipterygia (MepDyP; LDF). Homogeneous preparations of these DyPs were obtained after different steps of fast protein liquid chromatography, and they increase the total number of characterized fungal DyP proteins to eight. The peptide sequences of AauDyP, MepDyP, and EglDyP showed highest homologies (52–56 %) to the DyPs of M. scorodonius. Five out of the eight characterized fungal DyPs were used to evaluate their catalytic properties compared to classic fungal and plant heme peroxidases, namely lignin peroxidase of Phanerochaete chrysosporium (PchLiP; WRF), versatile peroxidase of Bjerkandera adusta (BadVP; WRF), and generic peroxidases of Coprinopsis cinerea (CiP) and Glycine max (soybean peroxidase = SBP). All DyPs tested possess unique properties regarding the stability at low pH values: 50–90 % enzymatic activity remained after 4-h exposition at pH 2.5, and the oxidation of nonphenolic aromatic substrates (lignin model compounds) was optimal below pH 3. Furthermore, all DyPs efficiently oxidized recalcitrant dyes (e.g., Azure B) as well as the phenolic substrate 2,6-dimethoxyphenol. Thus, DyPs combine features of different peroxidases on the functional level and may be part of the biocatalytic system secreted by fungi for the oxidation of lignin and/or toxic aromatic compounds.




Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucl Acids Res 25:3389–3402
Arora D (1986) Mushrooms demystified, 2nd edn. Ten Speed, Berkeley
Ashby MT (2008) Inorganic chemistry of defensive peroxidases in the human oral cavity. J Dent Res 87:900–914
Asther M, Vilter H, Kurek B, Meunier JC (1992) An improved method for the purification of lignin peroxidases from Phanerochaete chrysosporium INA-12: properties of two major isoforms. Int J Biochem 24:1377–1383
Ayala M, Roman R, Vazquez-Duhalt R (2007) A catalytic approach to estimate the redox potential of heme-peroxidases. Biochem Biophys Res Commun 357:804–808
Banci L, Camarero S, Martinez AT, Martinez MJ, Perez-Boada M, Pierattelli R, Ruiz-Duenas FJ (2003) NMR study of manganese(II) binding by a new versatile peroxidase from the white-rot fungus Pleurotus eryngii. J Biol Inorg Chem 8:751–760
Bollag JM, Leonowicz A (1984) Comparative studies of extracellular fungal laccases. Appl Environ Microbiol 48:849–854
Bollag JM, Sjoblad RD, Liu SY (1979) Characterization of an enzyme from Rhizoctonia praticola which polymerizes phenolic compounds. Can J Microbiol 25:229–233
Camarero S, Ibarra D, Martinez MJ, Martinez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Caramelo L, Martinez MJ, Martinez AT (1999) A search for ligninolytic peroxidases in the fungus Pleurotus eryngii involving α-keto-γ-thiomethylbutyric acid and lignin model dimers. Appl Environ Microbiol 65:916–922
Choinowski T, Blodig W, Winterhalter KH, Piontek K (1999) The crystal structure of lignin peroxidase at 1.70 A resolution reveals a hydroxy group on the Cβ of tryptophan 171: a novel radical site formed during the redox cycle. J Mol Biol 286:809–827
Chung N, Aust SD (1995) Inactivation of lignin peroxidase by hydrogen peroxide during the oxidation of phenols. Arch Biochem Biophys 316:851–855
Dunford HB (1999) Heme peroxidases. Wiley, New York
Faraco V, Piscitelli A, Sannia G, Giardina P (2007) Identification of a new member of the dye-decolorizing peroxidase family from Pleurotus ostreatus. World J Microbiol Biotechnol 23:889–893
Fernandez-Fueyo E, Ruiz-Duenas FJ, Miki Y, Martinez MJ, Hammel KE, Martinez AT (2012) Lignin-degrading peroxidases from genome of selective ligninolytic fungus Ceriporiopsis subvermispora. J Biol Chem 287:16903–16916
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martinez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Gorecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TK, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Duenas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719
Gillikin JW, Graham JS (1991) Purification and developmental analysis of the major anionic peroxidase from the seed coat of Glycine max. Plant Physiol 96:214–220
Hammel KE, Cullen D (2008) Role of fungal peroxidases in biological ligninolysis. Curr Opin Plant Biol 11:349–355
Hatakka A, Lundell TK, Tervilä-Wilo ALM, Brunow G (1991) Metabolism of non-phenolic β-O-4 lignin model compounds by the white-rot fungus Phlebia radiata. Appl Microbiol Biotechnol 36:270–277
Heinfling A, Ruiz-Duenas FJ, Martinez MJ, Bergbauer M, Szewzyk U, Martinez AT (1998) A study on reducing substrates of manganese-oxidizing peroxidases from Pleurotus eryngii and Bjerkandera adusta. FEBS Lett 428:141–146
Hibbett DS (2006) A phylogenetic overview of the Agaricomycotina. Mycologia 98:917–925
Hiner AN, Hernandez-Ruiz J, Rodriguez-Lopez JN, Garcia-Canovas F, Brisset NC, Smith AT, Arnao MB, Acosta M (2002) Reactions of the class II peroxidases, lignin peroxidase and Arthromyces ramosus peroxidase, with hydrogen peroxide. Catalase-like activity, compound III formation, and enzyme inactivation. J Biol Chem 277:26879–26885
Hofrichter M, Vares T, Kalsi M, Galkin S, Scheibner K, Fritsche W, Hatakka A (1999) Production of manganese peroxidase and organic acids and mineralization of 14C-labelled lignin (14C-DHP) during solid-state fermentation of wheat straw with the white rot fungus Nematoloma frowardii. Appl Environ Microbiol 65:1864–1870
Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T (2010) New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol 87:871–897
Ikehata K, Buchanan ID, Smith DW (2004) Extracellular peroxidase production by Coprinus species from urea-treated soil. Can J Microbiol 50:57–60
Johjima T, Ohkuma M, Kudo T (2003) Isolation and cDNA cloning of novel hydrogen peroxide-dependent phenol oxidase from the basidiomycete Termitomyces albuminosus. Appl Microbiol Biotechnol 61:220–225
Kersten PJ, Kalyanaraman B, Hammel KE, Reinhammar B, Kirk TK (1990) Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J 268:475–480
Kim SJ, Shoda M (1999) Purification and characterization of a novel peroxidase from Geotrichum candidum Dec 1 involved in decolorization of dyes. Appl Environ Microbiol 65:1029–1035
Kim SJ, Ishikawa K, Hirai M, Shoda M (1995) Characteristics of a newly isolated fungus, Geotrichum candidum Dec 1, which decolorizes various dyes. J Ferment Bioeng 79:601–607
Kim H, Cho D, Won K, Kim Y (2009) Inactivation of Coprinus cinereus peroxidase during the oxidation of various phenolic compounds originated from lignin. Enzyme Microb Technol 45:150–155
Kirk TK, Tien M, Kersten PJ, Mozuch MD, Kalyanaraman B (1986) Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol-β-aryl ether substructure of lignin. Biochem J 236:279–287
Kirkpatrick N, Palmer JM (1989) A natural inhibitor of lignin peroxidase activity from Phanerochaete chrysosporium, active at low pH and inactivated by divalent metal ions. Appl Microbiol Biotechnol 30:305–311
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liers C, Ullrich R, Pecyna MJ, Schlosser D, Hofrichter M (2007) Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzyme Microb Technol 41:785–793
Liers C, Bobeth C, Pecyna MJ, Ullrich R, Hofrichter M (2010) DyP-like peroxidases of the jelly fungus Auricularia auricula-judae oxidize nonphenolic lignin model compounds and high-redox potential dyes. Appl Microbiol Biotechnol 85:1869–1879
Lundell T, Wever R, Floris R, Harvey P, Hatakka A, Brunow G, Schoemaker H (1993) Lignin peroxidase L3 from Phlebia radiata. Pre-steady-state and steady-state studies with veratryl alcohol and a non-phenolic lignin model compound 1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol. Eur J Biochem 211:391–402
McEldoon JP, Pokora AR, Dordick JS (1995) Lignin peroxidase-type activity of soybean peroxidase. Enzyme Microb Technol 17:359–365
Miki Y, Ichinose H, Wariishi H (2010) Molecular characterization of lignin peroxidase from the white-rot basidiomycete Trametes cervina: a novel fungal peroxidase. FEMS Microbiol Lett 304:39–46
Morgenstern I, Klopman S, Hibbett DS (2008) Molecular evolution and diversity of lignin degrading heme peroxidases in the Agaricomycetes. J Mol Evol 66:243–257
Nüske J, Scheibner K, Dornberger U, Ullrich R, Hofrichter M (2002) Large scale production of manganese-peroxidase using agaric white-rot fungi. Enzyme Microb Technol 30:556–561
Ogola HJ, Kamiike T, Hashimoto N, Ashida H, Ishikawa T, Shibata H, Sawa Y (2009) Molecular characterization of a novel peroxidase from the cyanobacterium Anabaena sp. strain PCC 7120. Appl Environ Microbiol 75:7509–7518
Pecyna MJ, Ullrich R, Bittner B, Clemens A, Schubert R, Scheibner K, Hofrichter M (2009) Molecular characterization of aromatic peroxygenase from Agrocybe aegerita. Appl Microbiol Biotechnol 84:885–897
Peng J, Xu J (2011) RaptorX: exploiting structure information for protein alignment by statistical inference. Proteins 79(Suppl 10):161–171
Popp JL, Kirk TK (1991) Oxidation of methoxybenzenes by manganese peroxidase and by Mn3+. Arch Biochem Biophys 288:145–148
Pühse M, Szweda RT, Ma Y, Jeworrek C, Winter R, Zorn H (2009) Marasmius scorodonius extracellular dimeric peroxidase - exploring its temperature and pressure stability. Biochim Biophys Acta 1794:1091–1098
Ruiz-Dueñas FJ, Morales M, Garcia E, Miki Y, Martinez MJ, Martinez AT (2009) Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. J Exp Bot 60:441–452
Ruiz-Duenas FJ, Fernandez E, Martinez MJ, Martinez AT (2011) Pleurotus ostreatus heme peroxidases: an in silico analysis from the genome sequence to the enzyme molecular structure. C R Biol 334:795–805
Scheibner M, Hulsdau B, Zelena K, Nimtz M, de Boer L, Berger RG, Zorn H (2008) Novel peroxidases of Marasmius scorodonius degrade β-carotene. Appl Microbiol Biotechnol 77:1241–1250
Shimokawa T, Hirai M, Shoda M, Sugano Y (2008) Efficient dye decolorization and production of dye decolorizing enzymes by the basidiomycete Thanatephorus cucumeris Dec 1 in a liquid and solid hybrid culture. J Biosci Bioeng 106:481–487
Smith AT, Doyle WA, Dorlet P, Ivancich A (2009) Spectroscopic evidence for an engineered, catalytically active Trp radical that creates the unique reactivity of lignin peroxidase. Proc Natl Acad Sci U S A 106:16084–16089
Sugano Y (2009) DyP-type peroxidases comprise a novel heme peroxidase family. Cell Mol Life Sci 66:1387–1403
Sugano Y, Nakano R, Sasaki K, Shoda M (2000) Efficient heterologous expression in Aspergillus oryzae of a unique dye-decolorizing peroxidase, DyP, of Geotrichum candidum Dec 1. Appl Environ Microbiol 66:1754–1758
Sugano Y, Muramatsu R, Ichiyanagi A, Sato T, Shoda M (2007) DyP, a unique dye-decolorizing peroxidase, represents a novel heme peroxidase family: ASP171 replaces the distal histidine of classical peroxidases. J Biol Chem 282:36652–36658
Taboada-Puig R, Lú-Chau T, Moreira MT, Feijoo G, Martínez MJ, Lema JM (2011) A new strain of Bjerkandera sp. production, purification and characterization of versatile peroxidase. World J Microbiol Biotechnol 27:115–122. doi:10.1007/s11274-010-0435-2
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161:238–249
Ullrich R, Nüske J, Scheibner K, Spantzel J, Hofrichter M (2004) Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl Environ Microbiol 70:4575–4581
Ullrich R, Liers C, Schimpke S, Hofrichter M (2009) Purification of homogeneous forms of fungal peroxygenase. J Biotechnol 4:1619–1626
van Bloois E, Torres Pazmino DE, Winter RT, Fraaije MW (2009) A robust and extracellular heme-containing peroxidase from Thermobifida fusca as prototype of a bacterial peroxidase superfamily. Appl Microbiol Biotechnol 86:1419–1430
Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol 2:388–393
Welinder KG, Mauro JM, Norskov-Lauritsen L (1992) Structure of plant and fungal peroxidases. Biochem Soc Trans 20:337–340
Yoshida T, Tsuge H, Konno H, Hisabori T, Sugano Y (2011) The catalytic mechanism of dye-decolorizing peroxidase DyP may require the swinging movement of an aspartic acid residue. FEBS J 278:2387–2394
Zámocký M, Furtmueller PG, Bellei M, Battistuzzi G, Stadlmann J, Vlasits J, Obinger C (2009) Intracellular catalase/peroxidase from the phytopathogenic rice blast fungus Magnaporthe grisea: expression analysis and biochemical characterization of the recombinant protein. Biochem J 418:443–451
Zorn H, Langhoff S, Scheibner M, Berger RG (2003) Cleavage of β, β-carotene to flavor compounds by fungi. Appl Microbiol Biotechnol 62:331–336
Acknowledgment
The work has been partly funded by the European Union (integrated projects Biorenew and Peroxicats), the Deutsche Bundesstiftung Umwelt (DBU, project 13211-032 “Pilzliche Sekretome”), the DFG Priority Program 1374 “Infrastructure-Biodiversity-Exploratories” (HO 1961/4–1) (Deutsche Forschungsgemeinschaft, projects Fupers and Funwood), the Deutscher Akademischer Austauschdienst (DAAD, PPP 50151083), and the Bundesministerium für Bildung und Forschung (BMBF, VNM 09/014). We thank K. Piontek, D. Plattner, and E. Strittmatter for useful comments and their know-how in peroxidase crystal structures as well as our coworkers I. Kluge, M. Kinne, M. Poraj-Kobielska, S. Peter, C. Dolge, T. Arnstadt, D.H. Nghi, and K. Barková for their help in the lab and useful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 638 kb)
Rights and permissions
About this article
Cite this article
Liers, C., Pecyna, M.J., Kellner, H. et al. Substrate oxidation by dye-decolorizing peroxidases (DyPs) from wood- and litter-degrading agaricomycetes compared to other fungal and plant heme-peroxidases. Appl Microbiol Biotechnol 97, 5839–5849 (2013). https://doi.org/10.1007/s00253-012-4521-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4521-2