Abstract
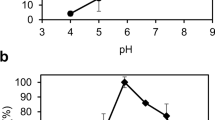
In this study, we report the characterization of a protein from Aspergillus oryzae, exhibiting sequence identity with paraben esterase from the genus Aspergillus. The coding region of 1,586 bp, including a 77-bp intron, encoded a protein of 502 amino acids. The gene without the signal peptide of 19 amino acids was cloned into a vector, pPICZαC, and expressed successfully in Pichia pastoris as an active extracellular protein. The purified recombinant protein had pH and temperature optima of 7.0–8.0 and 30 °C, respectively, and was stable at the pH range of 7.0–10.0 and up to 40 °C. The optimal substrate for hydrolysis by the purified recombinant protein, among a panel of α-naphthyl esters (C2–C16), was α-naphthyl butyrate (C4), with activity of 0.16 units/mg protein. The considerable hydrolytic activity of the purified recombinant enzyme toward tributyrin was determined. However, no paraben esterase activity was detected toward the ethyl, propyl, and butyl esters of 4-hydroxybenzoic acid. In addition, no activity was detected toward the methyl esters of ferulic, p-coumaric, caffeic, and sinapic acids that would indicate feruloyl esterase activity.






Similar content being viewed by others
References
Aguilar CN, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan LA, Ramírez-Coronel A, Contreras-Esquivel JC (2007) Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol 76:47–59
Benoit I, Danchin EGJ, Bleichrodt RJ, de Vries RP (2008) Biotechnological applications and potential of fungal feruloyl esterases based on prevalence, classification and biochemical diversity. Biotechnol Lett 30:387–396
Brenner S (1998) The molecular evolution of genes and proteins: a tale of two serines. Nature 334:528–530
Haag T, Loncrini DF (1984) Esters of para-hydroxybenzoic acid. Cosmet Sci Technol Ser 1:63–77
Hatamoto O, Watarai T, Kikuchi M, Mizusawa K, Sekine H (1996) Cloning and sequencing of the gene encoding tannase and a structural study of the tannase subunit from Aspergillus oryzae. Gene 175:215–221
Hotelier T, Renault L, Cousin X, Negre V, Marchot P, Chatonnet A (2004) Esther, the database of the α/β-hydrolase fold superfamily of proteins. Nucleic Acids Res 32:D145–D147
Kikuma T, Kitamoto K (2011) Analysis of autophagy in Aspergillus oryzae by disruption of Aoatg13, Aoatg4, and Aoatg15 genes. FEMS Microbiol Lett 316:61–69
Koseki T, Takahashi K, Fushinobu S, Iefuji H, Iwano K, Hashizume K, Matsuzawa H (2005) Mutational analysis of a feruloyl esterase from Aspergillus awamori involved in substrate discrimination and pH dependence. Biochim Biophys Acta 1722:200–208
Koseki T, Hori A, Seki S, Murayama T, Shiono Y (2009) Characterization of two distinct feruloyl esterases, AoFaeB and AoFaeC, from Aspergillus oryzae. Appl Microbiol Biotechnol 83:689–696
Koseki T, Mihara H, Murayama T, Shiono Y (2010) A novel Aspergillus oryzae esterase that hydrolyzes 4-hydroxybenzoic acid esters. FEBS Lett 584:4032–4036
Maeda H, Yamagata Y, Abe K, Hasegawa F, Machida M, Ishioka R, Gomi K, Nakajima T (2005) Purification and characterization of a biodegradable plastic-degrading enzyme from Aspergillus oryzae. Appl Microbiol Biotechnol 67:778–788
Ohnishi K, Yoshida Y, Toida J, Sekiguchi J (1994) Purification and characterization of a novel lipolytic enzyme from Aspergillus oryzae. J Ferment Bioeng 77:413–419
Ohnishi K, Toida J, Nakazawa H, Sekiguchi J (1995) Genomic structure and nucleotide sequence of a lipolytic enzyme gene of Aspergillus oryzae. FEMS Microbiol Lett 126:145–150
Olivares-Hernández R, Sunner H, Frisvad JC, Olsson L, Nielsen J, Panagiotou G (2010) Combining substrate specificity analysis with support vector classifiers reveals feruloyl esterase as a phylogenetically informative protein group. PLoS One 5:e12781
Saito N, Robert M, Kochi H, Matsuo G, Kakazu Y, Soga T, Tomita M (2009) Metabolic profiling reveals yihU as a novel hydroxybutyrate dehydrogenase for alternative succinic semialdehyde metabolism in Escherichia coli. J Biol Chem 284:16442–16451
Sundberg M, Poutanen K, Markkanen P, Linko M (1990) An extracellular esterase of Aspergillus awamori. Biotechnol Appl Biochem 12:670–680
Toida J, Kondoh K, Fukuzawa M, Ohnishi K, Sekiguchi J (1995) Purification and characterization of a lipase from Aspergillus oryzae. Biosci Biotechnol Biochem 59:1199–1203
Toida J, Arikawa Y, Kondoh K, Fukuzawa M, Sekiguchi J (1998) Purification and characterization of triacylglycerol lipase from Aspergillus oryzae. Biosci Biotechnol Biochem 62:759–763
Toida J, Fukuzawa M, Kobayashi G, Ito K, Sekiguchi J (2000) Cloning and sequencing of the triacylglycerol lipase gene of Aspergillus oryzae and its expression in Escherichia coli. FEMS Microbiol Lett 189:159–164
Tsuchiya A, Nakazawa H, Toida J, Ohnishi K, Sekiguchi J (1996) Cloning and nucleotide sequence of the mono- and diacylglycerol lipase gene (mdlB) of Aspergillus oryzae. FEMS Microbiol Lett 143:63–67
Udatha DBRKG, Mapelli V, Panagiotou G, Olsson L (2012) Common and distant structural characteristics of feruloyl esterase families from Aspergillus oryzae. PLoS One 7(6):e39473
Valkova N, Lépine F, Bollet C, Dupont M, Villemur R (2002) PrbA, a gene coding for an esterase hydrolyzing parabens in Enterobactor cloacae and Enterobactor gergoviae strains. J Bacteriol 184:5011–5017
Valkova N, Lépine F, Labrie L, Dupont M, Beaudet R (2003) Purification and characterization of PrbA, a new esterase from Enterobactor cloacae hydrolyzing the esters of 4-hydroxybenzoic acid (paraben). J Biol Chem 278:12779–12785
Zhong X, Peng L, Zheng S, Sun Z, Ren Y, Dong M, Xu A (2004) Secretion, purification, and characterization of a recombinant Aspergillus oryzae tannase in Pichia pastoris. Protein Expr Purif 36:165–169
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 15 kb)
Rights and permissions
About this article
Cite this article
Koseki, T., Asai, S., Saito, N. et al. Characterization of a novel lipolytic enzyme from Aspergillus oryzae . Appl Microbiol Biotechnol 97, 5351–5357 (2013). https://doi.org/10.1007/s00253-012-4391-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4391-7