Abstract
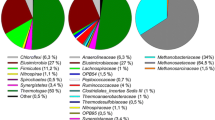
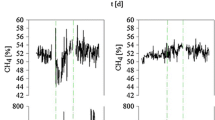
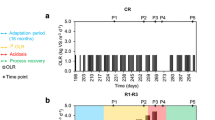
Agricultural biogas plants were operated in most cases below their optimal performance. An increase in the fermentation temperature and a spatial separation of hydrolysis/acetogenesis and methanogenesis are known strategies in improving and stabilizing biogas production. In this study, the dynamic variability of the bacterial and archaeal community was monitored within a two-phase leach bed biogas reactor supplied with rye silage and straw during a stepwise temperature increase from 55 to 75 °C within the leach bed reactor (LBR), using TRFLP analyses. To identify the terminal restriction fragments that were obtained, bacterial and archaeal 16S rRNA gene libraries were constructed. Above 65 °C, the bacterial community structure changed from being Clostridiales-dominated toward being dominated by members of the Bacteroidales, Clostridiales, and Thermotogales orders. Simultaneously, several changes occurred, including a decrease in the total cell count, degradation rate, and biogas yield along with alterations in the intermediate production. A bioaugmentation with compost at 70 °C led to slight improvements in the reactor performance; these did not persist at 75 °C. However, the archaeal community within the downstream anaerobic filter reactor (AF), operated constantly at 55 °C, altered by the temperature increase in the LBR. At an LBR temperature of 55 °C, members of the Methanobacteriales order were prevalent in the AF, whereas at higher LBR temperatures Methanosarcinales prevailed. Altogether, the best performance of this two-phase reactor was achieved at an LBR temperature of below 65 °C, which indicates that this temperature range has a favorable effect on the microbial community responsible for the production of biogas.





Similar content being viewed by others
References
Ahring BK (1995) Methanogenesis in thermophilic biogas reactors. Antonie Van Leeuwenhoek 67:91–102. doi:10.1007/BF00872197
Ahring BK, Ibrahim AA, Mladenovska Z (2001) Effect of temperature increase from 55 to 68 °C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res 35:2446–2452. doi:10.1016/S0043-1354(00)00526-1
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. J Water Pollut Control Fed 48:835–852
Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ (2005) At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl Environ Microbiol 71:7724–7736. doi:10.1128/AEM.71.12.7724-7736.2005
Ashelford KE, Chuzhanova NA, Fry JC, Jonas AJ, Weightman AJ (2006) New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl Environ Microbiol 72:5734–5741. doi:10.1128/AEM.00556-06
Bagi Z, Acs N, Balint B, Horvath L, Dobo K, Perei KR, Rakhely G, Kovacs KL (2007) Biotechnological intensification of biogas production. Appl Microbiol Biotechnol 76:473–482. doi:10.1007/s00253-007-1009-6
Buhr HO, Andrews JF (1977) The thermophilic anaerobic digestion process. Water Res 11:129–143. doi:10.1016/0043-1354(77)90118-X
Cai J, Wang Y, Liu D, Zeng Y, Xue Y, Ma Y, Feng Y (2007) Fervidobacterium changbaicum sp. nov., a novel thermophilic anaerobic bacterium isolated from a hot spring of the Changbai Mountains, China. Int J Syst Evol Microbiol 57:2333–2336. doi:10.1099/ijs.0.64758-0
Cirne DG, Lehtomäki A, Björnsson L, Blackall LL (2007) Hydrolysis and microbial community analyses in two-stage anaerobic digestion of energy crops. J Appl Microbiol 103:516–527. doi:10.1111/j.1365-2672.2006.03270.x
Clarke KR (1993) Nonparametric multivariate analyses of changes in community structure. Aust Ecol 18:117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Colwell RK (2006) EstimateS: statistical estimation of species richness and shared species from samples. Version 8.0.0. User’s Guide and application. http://purl.oclc.org/estimates. Accessed 4 Jan 2012
Culman SW, Bukowski R, Gauch HG, Cadillo-Quiroz H, Buckley DH (2009) Software open access T-REX: software for the processing and analysis of T-RFLP data. BMC Bioinformatics 10:171. doi:10.1186/1471-2105-10-171
Dahlenborg M, Borch E, Radström P (2001) Development of a combined selection and enrichment PCR procedure for Clostridium botulinum types B, E, and F and its use to determine prevalence in fecal samples from slaughtered pigs. Appl Environ Microbiol 67:4781–4788. doi:10.1128/AEM.67.10.4781-4788.2001
Dahlenborg M, Borch E, Radström P (2003) Prevalence of Clostridium botulinum types B, E and F in faecal samples from Swedish cattle. Int J Food Microbiol 82:105–110. doi:10.1016/S0168-1605(02)00255-6
Demirer GN, Chen S (2005) Two-phase anaerobic digestion of unscreened dairy manure. Process Biochem 40:3542–3549. doi:10.1016/j.procbio.2005.03.062
Després VR, Nowoisky JF, Klose M, Conrad R, Andreae MO, Pöschl U (2007) Characterization of primary biogenic aerosol particles in urban, rural, and high-alpine air by DNA sequence and restriction fragment analysis of ribosomal RNA genes. Biogeosciences 4:1127–1141
Dugba PN, Zhang RH (1999) Treatment of dairy wastewater with two-stage anaerobic sequencing batch reactor systems—thermophilic versus mesophilic operations. Bioresour Technol 68:225–233. doi:10.1016/S0960-8524(98)00156-4
Dunbar J, Ticknor LO, Kuske CR (2001) Phylogenetic specificity and reproducibility and new method for analysis of terminal restriction fragment profiles of 16S rRNA genes from bacterial communities. Appl Environ Microbiol 67:190–197. doi:10.1128/AEM.67.1.190-197.2001
Fernandez A, Huang SY, Seston S, Xing J, Hickey R, Criddle C, Tiedje J (1999) How stable is stable? Function versus community composition. Appl Environ Microbiol 65:3697–3704
Goodfellow M, Kämpfer P, Chun J, De Vos P, Rainey FA, Whitman WB (2010) Volume four: the Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes. In: Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB (eds) Bergey’s manual® of systematic bacteriology. Springer, New York, pp 1–931
Großkopf R, Janssen PH, Liesack W (1998) Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol 64:960–969
Kitts CL (2001) Terminal restriction fragment patterns: a tool for comparing microbial communities and assessing community dynamics. Curr Issues Intest Microbiol 2:17–25
Klocke M, Nettmann E, Bergmann I, Mundt K, Souidi K, Mumme J, Linke B (2008) Characterization of the methanogenic Archaea within two-phase biogas reactor systems operated with plant biomass. Syst Appl Microbiol 31:190–205. doi:10.1016/j.syapm.2008.02.003
Kongjan P, Thong S, Angelidaki I (2011) Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour Technol 102:4028–4035. doi:10.1016/j.biortech.2010.12.009
Krakat N, Westphal A, Satke K, Schmidt S, Scherer P (2010) The microcosm of a biogas fermenter: comparison of moderate hyperthermophilic (60°C) with thermophilic (55°C) conditions. Eng Life Sci 10:520–527. doi:10.1002/elsc.201000064
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–147
Lepistö R, Rintala J (1996) Conversion of volatile fatty acids in an extreme thermophilic (76–80°C) upflow anaerobic sludge-blanket reactor. Bioresour Technol 56:221–227
Lueders T, Friedrich M (2000) Archaeal population dynamics during sequential reduction processes in rice field soil. Appl Environ Microbiol 66:2732–2742. doi:10.1128/AEM.66.7.2732-2742.2000
Nettmann E, Bergmann I, Pramschüfer S, Mundt K, Plogsties V, Herrmann C, Klocke M (2010) Polyphasic analyses of methanogenic archaeal communities in agricultural biogas plants. Appl Environ Microbiol 76:2540–2548. doi:10.1128/AEM.01423-09
Neumann L, Scherer P (2011) Impact of bioaugmentation by compost on the performance and ecology of an anaerobic digester fed with energy crops. Bioresour Technol 102:2931–2935. doi:10.1016/j.biortech.2010.11.068
Nielsen HB, Mladenovska Z, Ahring BK (2007) Bioaugmentation of a two-stage thermophilic (68°C/55°C) anaerobic digestion concept for improvement of the methane yield from cattle manure. Biotechnol Bioeng 97:1638–1643. doi:10.1002/bit.21342
Nozhevnikova AN, Kotsyurbenko OR, Parshina SN (1999) Anaerobic manure treatment under extreme temperature conditions. Water Sci Technol 40:215–221. doi:10.1016/S0273-1223(99)00387-X
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov., a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch Microbiol 141:63–69
Rademacher A, Zakrzewski M, Schlüter A, Schönberg M, Szczepanowski R, Goesmann A, Pühler A, Klocke M (2012) Characterization of microbial biofilms in a thermophilic biogas system by high-throughput metagenome sequencing. FEMS Microbiol Ecol 79:785–799. doi:10.1111/j.1574-6941.2011.01265.x
Raizada N (2004) In: Berichte aus Wassergüte- und Abfallwirtschaft (ed) Application of molecular-biological method for optimization of anaerobic reactors. Technische Universität München, Garching
Schönberg M, Linke B (2012) The influence of the temperature regime on the formation of methane in a two-phase anaerobic digestion process. Eng Life Sci 12:1–8. doi:10.1002/elsc.201100062
Schutte UME, Abdo Z, Bent SJ, Shyu C, Williams CJ, Pierson JD, Forney LJ (2008) Advances in the use of terminal restriction fragment length polymorphism (T-RFLP) analysis of 16S rRNA genes to characterize microbial communities. Appl Microbiol Biotechnol 80:365–380. doi:10.1007/s00253-008-1565-4
Sipos R, Szekely AJ, Palatinszky M, Revesz S, Marialigeti K, Nikolausz M (2007) Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. FEMS Microbiol Ecol 60:341–350. doi:10.1111/j.1574-6941.2007.00283.x
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Wang H, Vuorela M, Keranen AL, Lehtinen TM, Lensu A, Lehtomaki A, Rintala J (2010) Development of microbial populations in the anaerobic hydrolysis of grass silage for methane production. FEMS Microbiol Ecol 72:496–506. doi:10.1111/j.1574-6941.2010.00850.x
Ward AJ, Hobbs PJ, Holliman PJ, Jones DL (2008) Optimisation of the anaerobic digestion of agricultural resources. Bioresour Technol 99:7928–7940. doi:10.1016/j.biortech.2008.02.044
Wasserfallen A, Nolling J, Pfister P, Reeve J, de Macario EC (2000) Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermautotrophicus comb. nov., Methanothermobacter wolfeii comb. nov., and Methanothermobacter marburgensis sp. nov. Int J Syst Evol Microbiol 50:43–53
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Wu W-M, Thiele JH, Jain MK, Zeikus JG (1993) Metabolic properties and kinetics of methanogenic granules. Appl Microbiol Biotechnol 39:804–811. doi:10.1007/BF00164470
Acknowledgments
Financial support for A. Rademacher and M. Schönberg was provided by the Federal Ministry of Education and Research (BMBF) through Project Management Jülich (PTJ, grant number 03SF0349C). The authors thank M. Jäkel, C. Jost, M. Felgentreu, C. Krumrei, J. Brunner, and K. Mundt for their valuable technical support. Dr. A. Ulrich, Leibniz Center for Agricultural Landscape Research (ZALF), is gratefully acknowledged for his valuable contributions to the discussion concerning TRFLP analysis and data interpretation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rademacher, A., Nolte, C., Schönberg, M. et al. Temperature increases from 55 to 75 °C in a two-phase biogas reactor result in fundamental alterations within the bacterial and archaeal community structure. Appl Microbiol Biotechnol 96, 565–576 (2012). https://doi.org/10.1007/s00253-012-4348-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4348-x