Abstract
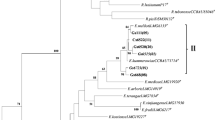
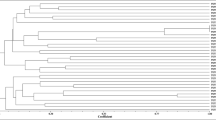
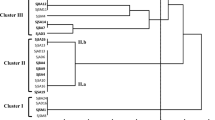
In search of effective nitrogen-fixing strains for inoculating Leucaena leucocephala, we assessed the symbiotic efficiency of 41 rhizobial isolates from root nodules of L. leucocephala growing in the arid–hot river valley area in Panxi, China. The genetic diversity of the isolates was studied by analyzing the housekeeping genes 16S rRNA and recA, and the symbiotic genes nifH and nodC. In the nodulation and symbiotic efficiency assay, only 11 of the 41 isolates promoted the growth of L. leucocephala while the majority of the isolates were ineffective in symbiotic nitrogen fixation. Furthermore, one fourth of the isolates had a growth slowing effect on the host. According to the 16S rRNA and recA gene analyses, most of the isolates were Ensifer spp. The remaining isolates were assigned to Rhizobium, Mesorhizobium and Bradyrhizobium. The sequence analyses indicated that the L. leucocephala rhizobia had undergone gene recombination. In contrast to the promiscuity observed as a wide species distribution of the isolates, the results implied that L. leucocephala is preferentially nodulated by strains that share common symbiosis genes. The symbiotic efficiency was not connected to chromosomal background of the symbionts and isolates carrying a similar nifH or nodC showed totally different nitrogen fixation efficiency.





Similar content being viewed by others
References
Aserse AA, Räsänen LA, Assefa F, Hailemariam A, Lindström K (2012) Phylogeny and genetic diversity of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. Syst Appl Microbiol 35:120–131. doi:10.1016/j.syapm.2011.11.005
Bala A, Giller KE (2001) Symbiotic specificity of tropical tree rhizobia for host legumes. New Phytol 149:495–507. doi:10.1046/j.1469-8137.2001.00059.x
Bala A, Giller KE (2006) Relationships between rhizobial diversity and host legume nodulation and nitrogen fixation in tropical ecosystems. Nutr Cycl Agroecosys 76:319–330. doi:10.1007/s10705-005-2003-y
Benata H, Mohammed O, Noureddine B, Abdelbasset B, Abdelmoumen H, Muresu R, Squartini A, El Idrissi MM (2008) Diversity of bacteria that nodulate Prosopis juliflora in the eastern area of Morocco. Syst Appl Microbiol 31:378–386. doi:10.1016/j.syapm.2008.08.002
Binde DR, Menna P, Bangel EV, Barcellos FG, Hungria M (2009) rep-PCR fingerprinting and taxonomy based on the sequencing of the 16S rRNA gene of 54 elite commercial rhizobial strains. Appl Microbiol Biotechnol 83:897–908. doi:10.1007/s00253-009-1927-6
Cardoso JD, Hungria M, Andrade DS (2012) Polyphasic approach for the characterization of rhizobial symbionts effective in fixing N2 with common bean (Phaseolus vulgaris L.). Appl Microbiol Biotechnol 93:2035–2049. doi:10.1007/s00253-011-3708-2
Chen Q, Zhang XP, Terefework Z, Kaifalainen S, Li DY, Lindström K (2003) Diversity and compatibility of peanut (Arachis hypogaea L.) bradyrhizobia and their host plants. Plant Soil 255:605–617. doi:10.1023/A:1026039503225
Debellé F, Moulin L, Mangin B, Dénarié J, Boivin C (2001) nod genes and Nod signals and the evolution of the Rhizobium legume symbiosis. Acta Biochim Pol 48:359–365
Diouf D, Fall D, Chaintreuil C, Ba AT, Dreyfus B, Neyra M, Ndoye I, Moulin L (2010) Phylogenetic analyses of symbiotic genes and characterization of functional traits of Mesorhizobium spp. strains associated with the promiscuous species Acacia seyal Del. J Appl Microbiol 108:818–830. doi:10.1111/j.1365-2672.2009.04500.x
Gaunt MW, Tumer SL, Rigottier-Gois L, Lloyd-MaeGlip SA, Young JPW (2001) Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int J Syst Evol Microbiol 51(6):2037–2048. doi:10.1099/00207713-51-6-2037
Gehlot HS, Panwar D, Tak N, Tak A, Sankhla IS, Poonar N, Parihar R, Shekhawat NS, Kumar M, Tiwari R, Ardley J, James EK, Sprent JI (2012) Nodulation of legumes from the Thar desert of India and molecular characterization of their rhizobia. Plant Soil. doi:10.1007/s11104-012-1143-5
Howieson JG, Yates RJ, Foster KJ, Real D, Besier RB (2008) Prospects for the future use of legumes. In: Dilworth MJ, James EK, Sprent JI, Newton WE (eds) Nitrogen fixing leguminous symbioses. Springer, Dordrecht, pp 363–393
Laguerre G, Nour SM, Macheret V, Sanjuan J, Drouin P, Amarger N (2001) Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiol 147:981–993
Laranjo M, Alexandre A, Rivas R, Velázquenz E, Young JPW, Oliveira S (2008) Chickpea rhizobia symbiosis genes are highly conserved across multiple Mesorhizobium species. FEMS Microbiol Ecol 66(2):391–400. doi:10.1111/j.1574-6941.2008.00584.x
Li QF, Zhang XP, Zou L, Chen Q, Fewer DP, Lindström K (2009) Horizontal gene transfer and recombination shape mesorhizobial populations in the gene center of the host plants Astragalus luteolus and Astragalus ernestii in Sichuan, China. FEMS Microbiol Ecol 70:227–235. doi:10.1111/j.1574-6941.2009.00776.x
Li QQ, Wang ET, Zhang YZ, Zhang YM, Tian CF, Sui XH, Chen YW, Chen YX (2011) Diversity and biogeography of rhizobia isolated from root nodules of Glycine max grown in Hebei Province, China. Microb Ecol 61:917–931. doi:10.1007/s00248-011-9820-0
Li L, Sinkko H, Montonen L, Wei G, Lindström K, Räsänen LA (2012) Biogeography of symbiotic and other endophytic bacteria isolated from medicinal Glycyrrhiza species in China. FEMS Microbiol Ecol 79:46–68. doi:10.1111/j.1574-6941.2011.01198.x
Liu XY, Wei S, Wang F, James EK, Guo XY, Zagar C, Xia LG, Dong X, Wang YP (2012) Burkholderia and Cupriavidus spp. are the preferred symbionts of Mimosa spp. in Southern China. FEMS Microbiol Ecol 80:417–426. doi:10.1111/j.1574-6941.2012.01310.x
Mnasri B, Badri Y, Saїdi S, de Lajudie P, Mhamdi R (2009) Symbiotic diversity of Ensifer meliloti strains recovered from various legume species in Tunisia. Syst Appl Microbiol 32:583–592. doi:10.1016/j.syapm.2009.07.007
Moawad H, Bohlool BB (1984) Competition among Rhizobium spp. for nodulation of Leucaena leucocephala in two tropical soils. Appl Environ Microbiol 48(1):5–9
Peoples MB, Herridge DF, Ladha JK (1995) Biological nitrogen fixation: an efficient source of nitrogen for sustainable agricultural production? Plant Soil 174:3–28. doi:10.1007/BF00032239
Raymond J, Siefert JL, Staples CR, Blankenship RE (2004) The natural history of nitrogen fixation. Mol Biol Evol 21(3):541–554. doi:10.1093/molbev/msh047
Rohlf FJ (1990) NTSYS-pc, Numerical taxonomy and multivariate analysis system, version 2.02. Exeter Software, Setauket, NY
Romdhane SB, Nasr H, Samba-Mbaye R, Neyra M, Ghorbal MH, De Lajudie P (2006) Genetic diversity of Acacia tortilis ssp. raddiana rhizobia in Tunisia assessed by 16S and 16S-23S rDNA genes analysis. J Appl Microbiol 100:436–445. doi:10.1111/j.1365-2672.2005.02765.x
Somasegaran P, Martin RB (1986) Symbiotic characteristics and Rhizobium requirements of a Leucaena leucocephala × Leucaena diversifolia hybrid and its parental genotypes. Appl Environ Microbiol 52(6):1422–1424
Sprent JI, Gehlot HS (2010) Nodulated legumes in arid and semi-arid environments: are they important? Plant Ecol Divers 3:211–219. doi:10.1080/17550874.2010.538740
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599. doi:10.1093/molbev/msm092
van Berkum P, Terefework Z, Paulin L, Suomalainen S, Lindström K, Eardly BD (2003) Discordant phylogenies within the rrn loci of rhizobia. J Bacteriol 185:2988–2998. doi:10.1128/JB.185.10.2988-2998.2003
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell, Oxford, UK
Wang ET, Martínez-Romero J, Martínez-Romero E (1999) Genetic diversity of rhizobia from Leucaena leucocephala nodules in Mexican soils. Mol Ecol 8:711–724. doi:10.1046/j.1365-294X.1999.00608.x
Wang FQ, Wang ET, Zhang YF, Chen WX (2006) Characterization of rhizobia isolated from Albizia spp. in comparison with microsymbionts of Acacia spp. and Leucaena leucocephala grown in China. Syst Appl Microbiol 29:502–517. doi:10.1016/j.syapm.2005.12.010
Wei G, Chen W, Zhu W, Chen C, Young JPW, Bontemps C (2009) Invasive Robinia pseudoacacia in China is nodulated by Mesorhizobium and Sinorhizobium species that share similar nodulation genes with native American symbionts. FEMS Microbiol Ecol 68:320–328. doi:10.1111/j.1574-6941.2009.00673.x
Weir BS, Turner SJ, Silvester WB, Park DC, Young JM (2004) Unexpectedly diverse Mesorhizobium strains and Rhizobium leguminosarum nodulate native legume genera of New Zealand, while introduced legume weeds are nodulated by Bradyrhizobium species. Appl Environ Microbiol 70:5980–5987. doi:10.1128/AEM.70.10.5980-5987.2004
Woffelman C (1994) DNAMAN for Windows, version 2.6. Lynon Biosoft, Institute of Molecular Plant Sciences. Leiden University, the Netherlands
Zhao L, Deng Z, Yang W, Yang W, Cao Y, Wang E, Wei G (2010) Diverse rhizobia associated with Sophora alopecuroides grown in different regions of Loess Plateau in China. Syst Appl Microbiol 33:468–477. doi:10.1016/j.syapm.2010.08.004
Zhao K, Penttinen P, Guan T, Xiao J, Chen Q, Xu J, Lindström K, Zhang L, Zhang X, Strobel GA (2011) The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr Microbiol 62:182–190. doi:10.1007/s00284-010-9685-3
Acknowledgments
This research was supported by National Natural Science Foundation of China (31070004), Educational Commission of Sichuan Province of China (09ZB047) and the Specialized Research Found for the Doctoral Program of Higher Education of China (20060626006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, K.W., Penttinen, P., Chen, Y.X. et al. Symbiotic efficiency and phylogeny of the rhizobia isolated from Leucaena leucocephala in arid–hot river valley area in Panxi, Sichuan, China. Appl Microbiol Biotechnol 97, 783–793 (2013). https://doi.org/10.1007/s00253-012-4246-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4246-2