Abstract
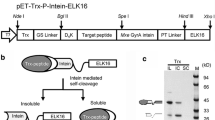
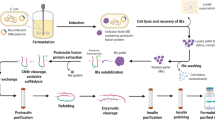
Glucagon-like peptide-1 as an endogenous glucose-lowering peptide is a promising candidate for anti-diabetic drug development. Here, we developed a convenient method by coupling of refolding and intein-mediated self-cleavage (CRIS) to improve the recombinant production of a mutated glucagon-like peptide-1 (mGLP-1). Bacterial cell culture employing auto-induction was performed at 37 °C to avoid the intracellular self-cleavage of the intein fusion protein. The impacts of urea, pH, and temperature on the efficiency of CRIS were tested, and then, the optimized CRIS was established. Using the optimized method, we obtained the purified mGLP-1 with a yield of 3.41 mg peptide/g bacterial cells which was 5.6-fold higher than before. After that, using chromatography, peptide electrophoresis, and mass spectrometry, we determined the purity and molecular weight of the purified peptide and then confirmed its glucose-lowering activity by performing glucose tolerance test in mice. These results suggest that CRIS is a relatively simple and efficacious method for the recombinant production of mGLP-1, and as a general method, it can also be used for the recombinant preparation of some other proteins and peptides.








Similar content being viewed by others
References
Banki MR, Gerngross TU, Wood DW (2005) Novel and economical purification of recombinant proteins: intein-mediated protein purification using in vivo polyhydroxybutyrate (PHB) matrix association. Protein Sci 14:1387–1395
Bernard MP, Cao D, Myers RV, Moyle WR (2004) Tight attachment of chitin-binding-domain-tagged proteins to surfaces coated with acetylated chitosan. Anal Biochem 327:278–283
Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368:1696–1705
Elleuche S, Poggeler S (2010) Inteins, valuable genetic elements in molecular biology and biotechnology. Appl Microbiol Biotechnol 87:479–489
Ferrandon S, Sterzenbach T, Mersha FB, Xu MQ (2003) A single surface tryptophan in the chitin-binding domain from Bacillus circulans chitinase A1 plays a pivotal role in binding chitin and can be modified to create an elutable affinity tag. Biochim Biophys Acta 1621:31–40
Fong BA, Wu WY, Wood DW (2009) Optimization of ELP-intein mediated protein purification by salt substitution. Protein Expr Purif 66:198–202
Freydell EJ, van der Wielen L, Eppink M, Ottens M (2010) Ion-exchange chromatographic protein refolding. J Chromatogr A 1217:7265–7274
Gao MM, Ma C, Liu WC, Zhu J, Tian H, Gao XD, Yao WB (2010a) Production and purification of an analog of glucagon-like peptide-1 by auto-induction and on-column cleavage in Escherichia coli. World J Microb Biot 26:1675–1682
Gao MM, Tian H, Ma C, Gao XD, Guo W, Yao WB (2010b) Expression, purification, and C-terminal site-specific PEGylation of cysteine-mutated glucagon-like peptide-1. Appl Biochem Biotechnol 162:155–165
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439
Hong J, Wang Y, Ye X, Zhang YH (2008) Simple protein purification through affinity adsorption on regenerated amorphous cellulose followed by intein self-cleavage. J Chromatogr A 1194:150–154
Jayakumar R, Prabaharan M, Nair SV, Tamura H (2010) Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol Adv 28:142–150
Li Y (2011) Self-cleaving fusion tags for recombinant protein production. Biotechnol Lett 33:869–881
Lu W, Sun Z, Tang Y, Chen J, Tang F, Zhang J, Liu JN (2011) Split intein facilitated tag affinity purification for recombinant proteins with controllable tag removal by inducible auto-cleavage. J Chromatogr A 1218:2553–2560
Ma C, Gao MM, Liu W, Zhu J, Tian H, Gao XD, Yao WB (2010) Intein-mediated expression and purification of an analog of glucagon-like peptide-1 in Escherichia coli. Protein Pept Lett 17:1245–1250
Nielsen LL (2005) Incretin mimetics and DPP-IV inhibitors for the treatment of type 2 diabetes. Drug Discov Today 10:703–710
Schagger H (2006) Tricine–SDS-PAGE. Nat Protoc 1:16–22
Schmoeger E, Berger E, Trefilov A, Jungbauer A, Hahn R (2009) Matrix-assisted refolding of autoprotease fusion proteins on an ion exchange column. J Chromatogr A 1216:8460–8469
Sharma SS, Chong S, Harcum SW (2006) Intein-mediated protein purification of fusion proteins expressed under high-cell density conditions in E. coli. J Biotechnol 125:48–56
Singh PK, Gupta MN (2008) Simultaneous refolding and purification of a recombinant lipase with an intein tag by affinity precipitation with chitosan. Biochim Biophys Acta 1784:1825–1829
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234
Wood DW, Wu W, Belfort G, Derbyshire V, Belfort M (1999) A genetic system yields self-cleaving inteins for bioseparations. Nat Biotechnol 17:889–892
Wu WY, Miller KD, Coolbaugh M, Wood DW (2011) Intein-mediated one-step purification of Escherichia coli secreted human antibody fragments. Protein Expr Purif 76:221–228
Xu MQ, Evans TC Jr (2001) Intein-mediated ligation and cyclization of expressed proteins. Methods 24:257–277
Yasuda M, Murakami Y, Sowa A, Ogino H, Ishikawa H (1998) Effect of additives on refolding of a denatured protein. Biotechnol Progr 14:601–606
Zhao Z, Lu W, Dun B, Jin D, Ping S, Zhang W, Chen M, Xu MQ, Lin M (2008) Purification of green fluorescent protein using a two-intein system. Appl Microbiol Biotechnol 77:1175–1180
Acknowledgments
This research was supported by the China National Nature Science Foundation (30973667, 81172974), the Jiangsu Province “Qing Lan Project” (2010), and the Perspective Research Foundation of Production Study and Research Alliance of Jiangsu Province of China (BY2011159).
Conflict of interest
The authors claim no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mingming Gao and Yue Tong contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, M., Tong, Y., Tian, H. et al. Recombinant production of mGLP-1 by coupling of refolding and intein-mediated self-cleavage (CRIS). Appl Microbiol Biotechnol 96, 1283–1290 (2012). https://doi.org/10.1007/s00253-012-4163-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4163-4