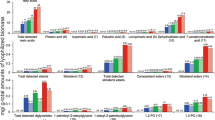
Abstract
As white-rot basidiomycetes, Phanerochaete species are critical to the cycling of carbon sequestered as woody biomass, and are predicted to encode many enzymes that can be harnessed to promote the conversion of lignocellulose to sugars for fermentation to fuels and chemicals. Advances in genomic, transcriptomic, and proteomic technologies have enabled detailed analyses of different Phanerochaete species and have revealed numerous enzyme families required for lignocellulose utilization, as well as insight into the regulation of corresponding genes. Recent studies of Phanerochaete are also exemplified by molecular analyses following cultivation on different wood preparations, and show substrate-dependent responses that were difficult to predict using model compounds or isolated plant polysaccharides. The aim of this mini-review is to synthesize results from studies that have applied recent advances in molecular tools to evaluate the expression and regulation of proteins that contribute to lignocellulose conversion in Phanerochaete species. The identification of proteins with as yet unknown function are also highlighted and noted as important targets for future investigation of white-rot decay.

Similar content being viewed by others
References
Abbas A, Koc H, Liu F, Tien M (2005) Fungal degradation of wood: initial proteomic analysis of extracellular proteins of Phanerochaete chrysosporium grown on oak substrate. Curr Genet 47:49–56
Alvarez JM, Canessa P, Mancilla RA, Polanco R, Santibanez PA, Vicuna R (2009) Expression of genes encoding laccase and manganese-dependent peroxidase in the fungus Ceriporiopsis subvermispora is mediated by an ACE1-like copper-fist transcription factor. Fungal Genet Biol 46:104–111
Aro N, Pakula T, Penttila M (2005) Transcriptional regulation of plant cell wall degradation by filamentous fungi. FEMS Microbiol Rev 29:719–739
Ayers AR, Ayers SB, Eriksson K-E (1978) Cellobiose oxidase, purification and partial characterization of a haemoprotein from Sporotrichum pulverulentum. Eur J Biochem 90:171–181
Bao W, Usha SN, Renganathan V (1993) Purification and characterization of cellobiose dehydrogenase, a novel extracellular hemo-flavoenzyme from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys 300:705–713
Belinky PA, Flikshtein N, Lechenko S, Gepstein S, Dosoretz CG (2003) Reactive oxygen species and induction of lignin peroxidase in Phanerochaete chrysosporium. Appl Environ Microbiol 69:6500–6506
Belinky PA, Flikshtein N, Dosoretz CG (2006) Induction of lignin peroxidase via reactive oxygen species in manganese-deficient cultures of Phanerochaete chrysosporium. Enzyme Microb Tech 39:222–228
Birch PRJ, Sims PFG, Broda P (1995) Substrate-dependent differential splicing of introns in the regions encoding the cellulose-binding domains of 2 exocellobiohydrolases-I-like genes in Phanerochaete chrysosporium. Appl Environ Microbiol 61:3741–3744
Boominathan K, Reddy CA (1992) cAMP-mediated differential regulation of lignin peroxidase and manganese-dependent peroxidase production in the white-rot Basidiomycete Phanerochaete chrysosporium. Proc Natl Acad Sci USA 89:5586–5590
Broda P, Birch PR, Brooks PR, Sims PF (1995) PCR-mediated analysis of lignocellulolytic gene transcription by Phanerochaete chrysosporium: substrate-dependent differential expression within gene families. Appl Environ Microbiol 61:2358–2364
Brown JA, Li D, Alic M, Gold MH (1993) Heat shock induction of manganese peroxidase gene transcription in Phanerochaete chrysosporium. Appl Environ Microbiol 59:4295–4299
Burdsall HH (1985) A contribution to the taxonomy of the genus Phanerochaete. Mycologia Memoirs 10:1–165
Burdsall HH, Eslyn W (1974) A new Phanerochaete with a Chrysosporium imperfect state. Mycotaxon 1:123–133
Cameron MD, Timofeevski S, Aust SD (2000) Enzymology of Phanerochaete chrysosporium with respect to the degradation of recalcitrant compounds and xenobiotics. Appl Microbiol Biotechnol 54:751–758
Canessa P, Alvarez JM, Polanco R, Bull P, Vicuna R (2008) The copper-dependent ACE1 transcription factor activates the transcription of the mco1 gene from the basidiomycete Phanerochaete chrysosporium. Microbiol-SGM 154:491–499
Covert SF, Vanden Wymelenberg A, Cullen D (1992) Structure, organization, and transcription of a cellobiohydrolase gene cluster from Phanerochaete chrysosporium. Appl Environ Microbiol 58:2168–2175
Dhawale SS (1993) Is an activator protein-2-like transcription factor involved in regulating gene expression during nitrogen limitation in fungi. Appl Environ Microbiol 59:2335–2338
Dittmer JK, Patel NJ, Dhawale SW, Dhawale SS (1997) Production of multiple laccase isoforms by Phanerochaete chrysosporium grown under nutrient sufficiency. FEMS Microbiol Lett 149:65–70
Durand H, Clanet M, Tiraby G (1988) Genetic improvement of Trichoderma reesei for large-scale cellulase production. Enzyme Microb Tech 10:341–346
Ebringerova A, Hromadkova Z, Heinze T (2005) Hemicellulose. Adv Polym Sci 186:1–67
Eriksson KE, Hamp SG (1978) Regulation of Endo-1,4-beta-glucanase production in Sporotrichum pulverulentum. Eur J Biochem 90:183–190
Freudenberg K (1965) Lignin: its constitution and formation from p-hydroxycinnamyl alcohols. Science 148:595–600
Galazka JM, Tian C, Beeson WT, Martinez B, Glass NL, Cate JH (2010) Cellodextrin transport in yeast for improved biofuel production. Science 330:84–86
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62:334–361
Gassara F, Brar SK, Tyagi RD, John RP, Verma M, Valero JR (2011) Parameter optimization for production of ligninolytic enzymes using agro-industrial wastes by response surface method. Biotechnol Bioproc E 16:343–351
Godfrey BJ, Mayfield MM, Brown JA, Gold MH (1990) Characterization of a gene encoding a manganese peroxidase from Phanerochaete chrysosporium. Gene 93:119–124
Gold MH, Alic M (1993) Molecular Biology of the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Microbiol Rev 57:605–622
Haemmerli SD, Schoemaker HE, Schmidt HWH, Leisola MSA (1987) Oxidation of veratryl alcohol by the lignin peroxidase of Phanerochaete chrysosporium: involvement of activated oxygen. FEBS Lett 220:149–154
Hammel KE, Cullen D (2008) Role of fungal peroxidases in biological ligninolysis. Curr Opin Plant Biol 11:349–355
Henrissat B, Driguez H, Viet C, Schulein M (1985) Synergism of cellulases from Trichoderma reesei in the degradation of cellulose. Biotechnology 3:722–726
Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T (2010) New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol 87(3):871–897
Holzbaur E, Tien M (1988) Structure and regulation of a lignin peroxidase gene from Phanerochaete chrysosporium. Biochem Biophys Res Commun 155:626–633
Hori C, Igarashi K, Katayama A, Samejima M (2011) Effects of xylan and starch on secretome of the basidiomycete Phanerochaete chrysosporium grown on cellulose. FEMS Microbiol Lett 321:14–23
Igarashi K, Samejima M, Eriksson KEL (1998) Cellobiose dehydrogenase enhances Phanerochaete chrysosporium cellobiohydrolase I activity by relieving product inhibition. Eur J Biochem 253:101–106
Janse BJH, Gaskell J, Akhtar M, Cullen D (1998) Expression of Phanerochaete chrysosporium genes encoding lignin peroxidases, manganese peroxidases, and glyoxal oxidase in wood. Appl Environ Microbiol 64:3536–3538
Kersten P, Cullen D (2007) Extracellular oxidative systems of the lignin-degrading Basidiomycete Phanerochaete chrysosporium. Fungal Genet Biol 44:77–87
Kim DW, Kim A, Kim RN, Nam SH, Kang A, Chung WT, Choi SH, Park HS (2010) Comparative analysis of expressed sequence tags from the white-rot fungi (Phanerochaete chrysosporium). Mol Cells 29:131–144
Kinne M, Poraj-Kobielska M, Ullrich R, Nousiainen P, Sipila J, Scheibner K, Hammel KE, Hofrichter M (2011) Oxidative cleavage of non-phenolic beta-O-4 lignin model dimers by an extracellular aromatic peroxygenase. Holzforschung 65:673–679
Kirk TK, Tien M, Faison BD (1984) Biochemistry of the oxidation of lignin by Phanerochaete chrysosproium. Biotechnol Adv 2:183–199
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth & Bisby’s Dictionary of the Fungi, 9th edn. CAB International, Wallingford
Kremer SM, Wood PM (1992a) Evidence that cellobiose oxidase from Phanerochete chrysosporiumis primarily an Fe(III) reductase. Eur J Biochem 205:133–138
Kremer SM, Wood PM (1992b) Production of Fenton's reagent by cellobiose oxidase from cellulolytic cultures of Phanerochaete chrysosporium. Eur J Biochem 208:807–814
Lamar RT, Larsen MJ, Kirk TK (1990) Sensitivity to and degradation of pentachlorophenol by Phanerochaete spp. Appl Environ Microbiol 56:3519–3526
Leisola MSA, Kozulic B, Meussdoerffer F, Fiechter A (1987) Homology among multiple extracellular peroxidases from Phanerochaete chrysosporium. J Biol Chem 262:419–424
Li D, Alic M, Brown JA, Gold MH (1995) Regulation of manganese peroxidase gene transcription by hydrogen peroxide, chemical stress, and molecular oxygen. Appl Environ Microbiol 61:341–345
Li B, Nagalla SR, Renganathan V (1996) Cloning of a cDNA encoding cellobiose dehydrogenase, a hemoflavoenzyme from Phanerochaete chrysosporium. Appl Environ Microbiol 62:1329–1335
Liers C, Bobeth C, Pecyna M, Ullrich R, Hofrichter M (2010) DyP-like peroxidases of the jelly fungus Auricularia auricula-judae oxidize nonphenolic lignin model compounds and high-redox potential dyes. Appl Microbiol Biotechnol 85:1869–1879
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Ma B, Mayfield MB, Godfrey BJ, Gold MH (2004) Novel promoter sequence required for manganese regulation of manganese peroxidase isozyme 1 gene expression in Phanerochaete chrysosporium. Eukaryot Cell 3:579–588
MacDonald J, Master ER (2012) Time-dependent profiles of transcripts encoding lignocellulose-modifying enzymes of the white rot fungus Phanerochaete carnosa grown on multiple wood substrates. Appl Environ Microbiol 78:1596–1600
MacDonald MJ, Ambler R, Broda P (1985) Regulation of intracellular cyclic AMP levels in the white-rot fungus Phanerochaete chrysosporium during the onset of idiophasic metabolism. Arch Microbiol 142:152–156
MacDonald J, Doering M, Canam T, Gong YC, Guttman DS, Campbell MM, Master ER (2011) Transcriptomic responses of the softwood-degrading white-rot fungus Phanerochaete carnosa during growth on coniferous and deciduous wood. Appl Environ Microbiol 77:3211–3218
Mach RL, Zeilinger S (2003) Regulation of gene expression in industrial fungi: Trichoderma. Appl Microbiol Biotechnol 60:515–522
Mahajan S, Master ER (2010) Proteomic characterization of lignocellulose-degrading enzymes secreted by Phanerochaete carnosa grown on spruce and microcrystalline cellulose. Appl Microbiol Biotechnol 86:1903–1914
Margolles-Clark E, Ilmen M, Penttila M (1997) Expression patterns of ten hemicellulase genes of the filamentous fungus Trichoderma reesei on various carbon sources. J Biotechnol 57:167–179
Martinez D, Larrondo L, Putnam N, Gelpke M, Huang K, Chapman J, Helfenbein KG, Ramaiya P, Detter JC, Larimer F, Coutinho PM, Henrissat B, Berka R, Cullen D, Rokhsar D (2004) Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol 22:695–700
Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC (2005) Biodegradation of lignocellulosics: microbial chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
Matityahu A, Hadar Y, Dosoretz CG, Belinky PA (2008) Gene silencing by RNA interference in the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol 74:5359–5365
Mayfield MB, Godfrey BJ, Gold MH (1994) Characterization of the mnp2 gene encoding manganese peroxidase isozyme-2 from the Basidiomycete Phanerochaete chrysosporium. Gene 142:231–235
Means AR (2000) Calcium and calmodulin-mediated regulatory mechanisms. In: Conn PM, Means AR (eds) Principles of molecular regulation. Humana Press, Totowa, pp 187–204
Minami M, Kureha O, Mori M, Kamitsuji H, Suzuki K, Irie T (2007) Long serial analysis of gene expression for transcriptome profiling during the initiation of ligninolytic enzymes production in Phanerochaete chrysosporium. Appl Microbiol Biotechnol 75:609–618
Minami M, Suzuki K, Shimizu A, Hongo T, Sakamoto T, Ohyama N, Kitaura H, Kusaka A, Iwama K, Irie T (2009) Changes in the gene expression of the white rot fungus Phanerochaete chrysosporium due to the addition of atropine. Biosci Biotech Bioch 73:1722–1731
Nogawa M, Goto M, Okada H, Morikawa Y (2001) l-sorbose induces cellulase gene transcription in the cellulolytic fungus Trichoderma reesei. Curr Genet 38:329–334
Ohnishi Y, Nagase M, Ichiyanagi T, Kitamoto Y, Aimi T (2007a) Transcriptional regulation of two cellobiohydrolase encoding genes (cel1 and cel2) from the wood-degrading basidiomycete Polyporus arcularius. Appl Microbiol Biotechnol 76:1069–1078
Ohnishi Y, Nagase M, Ichiyanagi T, Kitamoto Y, Aimi T (2007b) Transcriptional regulation of two endoglucanase-encoding genes (cel3A and cel4) from the wood-degrading basidiomycete Polyporus arcularius. FEMS Microbiol Lett 274:218–225
Phillips CM, Beeson WT, Cate JH, Marletta MA (2011) Cellobiose dehydrogenase and a copper-dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa. ACS Chem Biol 6(12):1399–1406
Podgornik H, Stegu M, Zibert E, Perdih A (2001) Laccase production by Phanerochaete chrysosporium—an artefact caused by Mn(III)? Lett Appl Microbiol 32:407–411
Polanco R, Canessa P, Rivas A, Larrondo LF, Lobos S, Vicuna R (2006) Cloning and functional characterization of the gene encoding the transcription factor Ace1 in the basidiomycete Phanerochaete chrysosporium. Biol Res 39:641–648
Ravalason H, Jan G, Molle D, Pasco M, Coutinho PM, Lapierre C, Pollet B, Bertaud F, Petit-Conil M, Grisel S, Sigoillot JC, Asther M, Herpoel-Gimbert I (2008) Secretome analysis of Phanerochaete chrysosporium strain CIRM-BRFM41 grown on softwood. Appl Microbiol Biotechnol 80:719–733
Rodriguez-Rincon F, Suarez A, Lucas M, Larrondo LF, de la Rubia T, Polaina J, Martinez J (2010) Molecular and structural modeling of the Phanerochaete flavido-alba extracellular laccase reveals its ferroxidase structure. Arch Microbiol 192:883–892
Sakamoto T, Kitaura H, Minami M, Honda Y, Watanabe T, Ueda A, Suzuki K, Irie T (2010) Transcriptional effect of a calmodulin inhibitor, W-7, on the ligninolytic enzyme genes in Phanerochaete chrysosporium. Curr Genet 56:401–410
Sakamoto T, Yao Y, Hida Y, Honda Y, Watanabe T, Hashigaya W, Suzuki K, Irie T (2012) A calmodulin inhibitor, W-7 influences the effect of cyclic adenosine 3′, 5′-monophosphate signaling on ligninolytic enzyme gene expression in Phanerochaete chrysosporium. AMB Express 2(1):7. doi:10.1186/2191-0855-2-7
Sato S, Liu F, Koc H, Tien M (2007) Expression analysis of extracellular proteins from Phanerochaete chrysosporium grown on different liquid and solid substrates. Microbiol-SGM 153:3023–3033
Sato S, Feltus FA, Iyer P, Tien M (2009) The first genome-level transcriptome of the wood-degrading fungus Phanerochaete chrysosporium grown on red oak. Curr Genet 55:273–286
Sharma RK, Kalra J (1994) Molecular interaction between cAMP and calcium in calmodulin-dependent cyclic-nucleotide phosphodiesterase system. Clin Invest Med 17:374–382
Shary S, Kapich AN, Panisko EA, Magnuson JK, Cullen D, Hammel KE (2008) Differential expression in Phanerochaete chrysosporium of membrane-associated proteins relevant to lignin degradation. Appl Environ Microbiol 74:7252–7257
Sims P, James C, Broda P (1988) The identification, molecular cloning and characterization of a gene from Phanerochaete chrysosporium that shows strong homology to the exo-cellobiohydrolase I gene from Trichoderma reesei. Gene 74:411–422
Singh D, Zeng J, Chen S (2011) Increasing manganese peroxidase productivity of Phanerochaete chrysosporium by optimizing carbon sources and supplementing small molecules. Lett Appl Microbiol 53:120–123
Sjostrom E (1993) Wood chemistry: fundamentals and applications. Academic, San Diego
Srinivasan C, D'Sousa TM, Boominathan K, Reddy CA (1995) Demonstration of laccase in the white rot Basidiomycete Phanerochaete chrysosporium BKM-F1767. Appl Environ Microbiol 61:4274–4277
Stewart P, Cullen D (1999) Organization and differential regulation of a cluster of lignin peroxidase genes of Phanerochaete chrysosporium. J Bacteriol 181:3427–3432
Stewart P, Kersten P, Vanden Wymelenberg A, Gaskell J, Cullen D (1992) Lignin peroxidase gene family of Phanerochaete chrysosporium: complex regulation by carbon and nitrogen limitation and identification of a second dimorphic chromosome. J Bacteriol 174:5036–5042
Stewart P, Gaskell J, Cullen D (2000) A homokaryotic derivative of a Phanerochaete chrysosporium strain and its use in genomic analysis of repetitive elements. Appl Environ Microbiol 66:1629–1633
Stricker AR, Mach RL, de Graaff LH (2008) Regulation of transcription of cellulases- and hemicellulases-encoding genes in Aspergillus niger and Hypocrea jecorina (Trichoderma reesei). Appl Microbiol Biotechnol 78:211–220
Suzuki H, Igarashi K, Samejima M (2008) Real-time quantitative analysis of carbon catabolite derepression of cellulolytic genes expressed in the basidiomycete Phanerochaete chrysosporium. Appl Microbiol Biotechnol 80:99–106
Suzuki H, Igarashi K, Samejima M (2009) Quantitative transcriptional analysis of the genes encoding glycoside hydrolase family 7 cellulase isozymes in the basidiomycete Phanerochaete chrysosporium. FEMS Microbiol Lett 299:159–165
Suzuki H, Igarashi K, Samejima M (2010) Cellotriose and cellotetraose as inducers of the genes encoding cellobiohydrolases in the Basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 76:6164–6170
Tempelaars CA, Birch PR, Sims PF, Broda P (1994) Isolation, characterization, and analysis of the expression of the cbhII gene of Phanerochaete chrysosporium. Appl Environ Microbiol 60:4387–4393
Tien M, Kirk TK (1983) Lignin-degrading enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science 221:661–663
Tien M, Kirk TK (1984) Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci U S A 81:2280–2284
Vaheri MP, Vaheri MEO, Kauppinen VS (1979) Formation and release of cellulolytic enzymes during growth of Trichoderma reesei on cellobiose and glycerol. Eur J Appl Microbiol 8:73–80
van den Brink J, de Vries RP (2011) Fungal enzyme sets for plant polysaccharide degradation. Appl Microbiol Biotechnol 91:1477–1492
Vanden Wymelenberg A, Covert S, Cullen D (1993) Identification of the gene encoding the major cellobiohydrolase of the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol 59:3492–3494
Vanden Wymelenberg A, Sabat G, Martinez D, Rajangam AS, Teeri TT, Gaskell J, Kersten PJ, Cullen D (2005) The Phanerochaete chrysosporium secretome: database predictions and initial mass spectrometry peptide identifications in cellulose-grown medium. J Biotechnol 118:17–34
Vanden Wymelenberg A, Gaskell J, Mozuch M, Kersten P, Sabat G, Martinez D, Cullen D (2009) Transcriptome and secretome analyses of Phanerochaete chrysosporium reveal complex patterns of gene expression. Appl Environ Microbiol 75:4058–4068
Vanden Wymelenberg A, Gaskell J, Mozuch M, Sabat G, Ralph J, Skyba O, Mansfield SD, Blanchette RA, Martinez D, Grigoriev I, Kersten PJ, Cullen D (2010) Comparative transcriptome and secretome analysis of wood decay fungi Postia placenta and Phanerochaete chrysosporium. Appl Environ Microbiol 76:3599–3610
Vanden Wymelenberg A, Gaskell J, Mozuch M, BonDurant SS, Sabat G, Ralph J, Skyba O, Mansfield SD, Blanchette RA, Grigoriev IV, Kersten PJ, Cullen D (2011) Significant alteration of gene expression in wood decay fungi Postia placenta and Phanerochaete chrysosporium by plant species. Appl Environ Microbiol 77:4499–4507
Whetten R, Sederoff R (1995) Lignin biosynthesis. Plant Cell 7:1001–1013
Wood TM (1992) Fungal cellulases. Biochem Soc Trans 20:46–53
Wu JM, Zhang YZ (2010) Gene expression in secondary metabolism and metabolic switching phase of Phanerochaete chrysosporium. Appl Biochem Biotechnol 162:1961–1977
Yoshida M, Igarashi K, Wada M, Kaneko S, Suzuki N, Matsumura H, Nakamura N, Ohno H, Samejima M (2005) Characterization of carbohydrate-binding cytochrome b(562) from the white-rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol 71:4548–4555
Acknowledgments
J. MacDonald and H. Suzuki were supported by grants from the Natural Sciences and Engineering Research Council to E.R.M. We thank Mr. A. Nakamura (University of Tokyo) for sharing his insight into Phanerochaete strain history.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacDonald, J., Suzuki, H. & Master, E.R. Expression and regulation of genes encoding lignocellulose-degrading activity in the genus Phanerochaete . Appl Microbiol Biotechnol 94, 339–351 (2012). https://doi.org/10.1007/s00253-012-3937-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-3937-z