Abstract
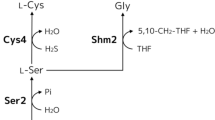
Glutathione (GSH) is a valuable tri-peptide that is widely used in the pharmaceutical, food, and cosmetic industries. Glutathione is produced industrially by fermentation using Saccharomyces cerevisiae. In this study, we demonstrated that engineering in sulfate assimilation metabolism can significantly improve GSH production. The intracellular GSH content of MET14 and MET16 over-expressing strains increased up to 1.2 and 1.4-fold higher than that of the parental strain, respectively, whereas those of APA1 and MET3 over-expressing strains decreased. Especially, in the MET16 over-expressing strain, the volumetric GSH concentration was up to 1.7-fold higher than that of the parental strain as a result of the synergetic effect of the increases in the cell concentration and the intracellular GSH content. Additionally, combinatorial mutant strains that had been engineered to contain both the sulfur and the GSH synthetic metabolism synergistically increased the GSH production. External addition of cysteine to S. cerevisiae is well known as a way to increase the intracellular GSH content; however, it results a decrease in cell growth. This study showed that the engineering of sulfur metabolism in S. cerevisiae proves more valuable than addition of cysteine as a way to boost GSH production due to the increases in both the intracellular GSH content and the cell growth.




Similar content being viewed by others
References
Alfafara CG, Kanda A, Shioi T, Shimizu H, Shioya S, Suga K (1992) Effect of amino acids on glutathione production by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 36:538–540
Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R (2005) Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol 204:238–255
Chen DC, Yang BC, Kuo TT (1992) One-step transformation of yeast in stationary phase. Curr Genet 21:83–84
Dröge W, Breitkreutz R (2000) Glutathione and immune function. Proc Nutr Soc 59:595–600
Flohé L (1985) The glutathione peroxidase reaction: molecular basis of the antioxidant function of selenium in mammals. Curr Top Cell Regul 27:473–478
Ganguli D, Kumar C, Bachhawat AK (2007) The alternative pathway of glutathione degradation is mediated by a novel protein complex involving three new genes in Saccharomyces cerevisiae. Genetics 175:1137–1151
Hara KY, Kim S, Yoshida H, Kiriyama K, Kondo T, Okai N, Ogino C, Fukuda H and Kondo A (2011) Development of a glutathione production process from proteinaceous biomass resources using protease-displaying Saccharomyces cerevisiae. Appl Microbiol Biotechnol. in press
Ishii J, Izawa K, Matsumura S, Wakamura K, Tanino T, Tanaka T, Ogino C, Fukuda H, Kondo A (2009) A simple and immediate method for simultaneously evaluating expression level and plasmid maintenance in yeast. J Biochem 145:701–708
Li Y, Wei G, Chen J (2004) Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol 66:233–242
Meister A, Anderson ME (1983) Glutathione Annu Rev Biochem 52:711–760
Murguía JR, Bellés JM, Serrano R (1996) The yeast HAL2 nucleotidase is an in vivo target of salt toxicity. J Biol Chem 271:29029–29033
Ohtake Y, Watanabe K, Tezuka H, Ogata T, Yabuuchi S, Murata K, Kimura A (1988) The expression of γ-glutamylcysteine synthetase gene of Escherichia coli B in Saccharomyces cerevisiae. Agric Biol Chem 52:2753–2762
Ohtake Y, Watanabe K, Tezuka H, Ogata T, Yabuuchi S, Murata K, Kimura A (1989) Expression of glutathione synthetase gene of Escherichia coli B in Saccharomyces cerevisiae. J Ferment Bioeng 68:390–399
Penninckx MJ (2000) A short review on the role of glutathione in the response of yeasts to nutritional, environmental, and oxidative stresses. Enzyme Microb Technol 26:737–742
Ray S, Watkins DN, Misso NL, Thompson PJ (2002) Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin Exp Allergy 32:571–577
Rennenberg H, Herschbach C, Haberer K, Kopriva S (2007) Sulfur metabolism in plants: are trees different? Plant Biol 9:620–637
Rolseth V, Djurhuus R, Svardal AM (2002) Additive toxicity of limonene and 50% oxygen and the role of glutathione in detoxification in human lung cells. Toxicology 170:75–88
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Singh RJ (2002) Glutathione: a marker and antioxidant for aging. J Lab Clin Med 140:380–381
Suzuki T, Yokoyama A, Tsuji T, Ikeshima E, Nakashima K, Ikushima S, Kobayashi C, Yoshida S (2011) Identification and characterization of genes involved in glutathione production in yeast. J Biosci Bioeng 112:107–113
Thomas D, Surdin-Kerjan Y (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61:503–532
Vartanyan LS, Gurevich S, Kozachenko AI, Nagler LG, Lozovskaya EL, Burlakova EB (2000) Changes in superoxide production rate and in superoxide dismutase and glutathione peroxidase activities in subcellular organelles in mouse liver under exposure to low doses of low-intensity radiation. Biochem Mosc 65:442–446
Wei G, Li Y, Du G, Chen J (2003) Effect of surfactants on extracellular accumulation of glutathione by Saccharomyces cerevisiae. Process Biochem 38:1133–1138
Wen S, Zhang T, Tana T (2004) Utilization of amino acids to enhance glutathione production in Saccharomyces cerevisiae. Enz Microbiol Technol 35:501–507
Yamada R, Taniguchi N, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Cocktail delta-integration: a novel method to construct cellulolytic enzyme expression ratio-optimized yeast strains. Microb Cell Fact 9:32
Yoshida K, Hariki T, Inoue H, Nakamura T (2002) External skin preparation for whitening. JP Patent 2, 002, 284, 664
Yoshida H, Hara KY, Kiriyama K, Nakayama H, Okazaki F, Matsuda F, Ogino C, Fukuda H, Kondo A (2011) Enzymatic glutathione production using metabolically engineered Saccharomyces cerevisiae as a whole-cell biocatalyst. Appl Microbiol Biotechnol 91:1001–1006
Acknowledgments
We are grateful to Dr. J. Ishii (Organization of Advanced Science and Technology, Kobe University) for providing us with pGK402, pRS405, and pRS406 plasmids. We thank Dr. R. Yamada (Organization of Advanced Science and Technology, Kobe University) for providing us with the δ-integration plasmid. This study was supported by the Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe, iBioK), MEXT, Japan. Hara KY was supported by a Grant-in-Aid for Young Scientists (B) (22760608).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kiyotaka Y. Hara and Kentaro Kiriyama contributed equally in this work.
Rights and permissions
About this article
Cite this article
Hara, K.Y., Kiriyama, K., Inagaki, A. et al. Improvement of glutathione production by metabolic engineering the sulfate assimilation pathway of Saccharomyces cerevisiae . Appl Microbiol Biotechnol 94, 1313–1319 (2012). https://doi.org/10.1007/s00253-011-3841-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3841-y