Abstract
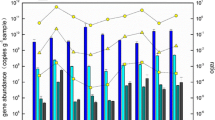
With the rapid development of ammonia-synthesizing industry, the ammonia-nitrogen pollution in wetlands acting as the sink of point and diffuse pollution has been increased dramatically. Most of ammonia-nitrogen is oxidized at least once by ammonia-oxidizing prokaryotes to complete the nitrogen cycle. Current research findings have expanded the known ammonia-oxidizing prokaryotes from the domain Bacteria to Archaea. However, in the complex wetlands environment, it remains unclear whether ammonia oxidation is exclusively or predominantly linked to Archaea or Bacteria as implied by specific high abundance. In this research, the abundance and composition of Archaea and Bacteria in sediments of four kinds of wetlands with different nitrogen concentration were investigated by using quantitative real-time polymerase chain reaction, cloning, and sequencing approaches based on amoA genes. The results indicated that AOA distributed widely in wetland sediments, and the phylogenetic tree revealed that archaeal amoA functional gene sequences from wetlands sediments cluster as two major evolutionary branches: soil/sediment and sediment/water. The bacteria functionally dominated microbial ammonia oxidation in different wetlands sediments on the basis of molecule analysis, potential nitrification rate, and soil chemistry. Moreover, the factors influencing AOA and AOB abundances with environmental indicator were also analyzed, and the results addressed the copy numbers of archaeal and bacterial amoA functional gene having the higher correlation with pH and ammonia concentration. The pH had relatively great negative impact on the abundance of AOA and AOB, while ammonia concentration showed positive impact on AOB abundance only. These findings could be fundamental to improve understanding of the importance of AOB and AOA in nitrogen and other nutrients cycle in wetland ecosystems.


Similar content being viewed by others
References
Anne ET, Peter JB (2006) Nitrite production by Nitrosomonas europaea and Nitrosospira sp. AV in soils at different solution concentrations of ammonium. Soil Biol Biochem 38(4):828–836
Avrahami S, Conrad R, Braker G (2002) Effect of soil ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl Environ Microbiol 68(11):5685–5692
Bao SD (2000) Soil and Agricultural Chemistry Analysis (3th Eidtion). China Agriculture, Beijing
Caffrey J, Bano N, Kalanetra K, Hollibaugh J (2007) Ammonia oxidation and ammonia-oxidizing bacteria and Archaea from estuaries with differing histories of hypoxia. ISME J 1:660–662
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not Archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia oxidizing Archaea. FEMS Microbiol Rev 33(5):855–869
Fan GN, Zhu GB, Wang Y, Wang SY, Wang CX, Yin CQ (2010) New functional microorganisms in nitrogen cycle restoration of river riparian ecosystems. Acta Scientiae Circumstantiae 30(8):1558–1563
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing Archaea in water columns and sediments of the ocean. Proceedings of the National Academy of Sciences 102(41):14683–14688
Gruber N, Galloway JN (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Hatzenpichler R, Lebedeva EV, Spieck E, Stoecker K, Richter A, Daims H, Wagner M (2008) A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci USA 105(6):2134–2139
He J, Shen J, Zhang L, Zhu Y, Zheng Y, Xu M, Di HJ (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing Archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9(9):2364
Herrmann M, Saunders AM, Schramm A (2008) Archaea dominate the ammonia-oxidizing community in the rhizosphere of the freshwater macrophyte Littorella uniflora. Appl Environ Microbiol 74(10):3279–3283
Jia ZJ, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11(7):1658–1671
Jiang H, Dong H, Yu B, Lv G, Deng S, Berzins N, Dai M (2009) Diversity and abundance of ammonia-oxidizing Archaea and bacteria in Qinghai Lake, northwestern China. Geomicrobiol J 26:199–211
Könneke M, Bernhard A, de la Torre JR, Walker CB, Waterbury JB, Stahl DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437(22):543–546
Kurola J, Salkinoja-Salonen M, Aarnio T, Hultman J, Romantschuk M (2005) Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiol Lett 250:33–38
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–979
Mosier A, Francis C (2008) Relative abundance and diversity of ammonia-oxidizing Archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10:3002–3016
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, David L, Scow KM (2004) Application of real-time PCR to study effects of ammonium on population size of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 70(2):1008–1016
Oved T, Shaviv A, Goldrath T, Mandelbaum RT, Minz D (2001) Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 67(8):3426–3433
Park HD, Wells GF, Bae H, Criddle CS, Francis CA (2006) Occurrence of ammonia-oxidizing Archaea in wastewater treatment plant bioreactors. Appl Environ Microbiol 72(8):5643–5647
Peng Yongzhen and Zhu Guibing (2006) Biological nitrogen removal with nitrification and denitrification via nitrite pathway. Appl Microbiol Biotechnol 73(1):15–26
Prosser JI (1989) Autotrophic nitrification in bacteria. Adv Microb Physiol 30:125–181
Prosser JI, Nicol GW (2008) Relative contributions of Archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10(11):2931–2941
Santoro A, Francis C, de Sieyes N, Boehm A (2008) Shifts in the relative abundance of ammonia-oxidizing bacteria and Archaea across physicochemical gradients in a subterranean estuary. Environ Microbiol 10:1068–1079
Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506
Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ (2008) Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing Archaea communities of an alkaline sandy loam. Environ Microbiol 10(6):1601–1611
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTALX windows interface: flexible strate-gies for multiple sequence alignment aided by quality analysistools. Nucleic Acids Res 25:4876–4882
Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA, Wu D, Paulsen I, Nelson KE, Nelson W, Fouts DE, Levy S, Knap AH, Lomas MW, Nealson K, White O, Peterson J, Hoffman J, Parsons R, Baden-Tillson H, Pfannkoch C, Rogers YH, Smith HO (2004) Environmental genome shotgun sequencing of the Sargasso sea. Science 304:66–74
Verhoeven JTA, Arheimer B, Yin C, Hefting MM (2006) Regional and global concerns over wetlands and water quality. Trends Ecol Evol 21(2):96–103
Wang H, Wang W, Yin C, Wang Y, Lu J (2006) Littoral zone as the “hotspots” of nitrous oxide (N2O) emission in a hyper-eutrophic lake in China. Atmos Environ 40:5522–5527
Wang H, Yang L, Wang W, Lu J, Yin C (2007) Nitrous oxide (N2O) fluxes and their relationship with water-sediment parameters in a hyper-eutrophic shallow Lake, China. J Geophys Res 112:G01005. doi:10.1029/2005JG000129
Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S, Damste JSS (2006) Archaeal nitrification in the ocean. Proceedings of the National Academy of Sciences 103(33):12317–12322
Zhang CL, Ye Q, Huang Z, Li W, Chen J, Song Z, Zhao W, Bagwell C, Inskeep WP, Ross C, Gao L, Wiegel J, Romanek CS, Shock EL, Hedlund BP (2008) Global occurrence of archaeal amoA genes in terrestrial hot springs. Appl Environ Microbiol 74(20):6417–6426
Zhang L, Wang M, Prosser J, Zheng Y, He J (2009) Altitude ammonia-oxidizing bacteria and Archaea in soils of Mount Everest. FEMS Microbiol Ecol 70:52–61
Zhu G, Peng Y, Li B, Guo J, Yang Q, Wang S (2008) Biological removal of nitrogen from wastewater. Reviews of Environmental Contamination and Toxicology. 159–195.
Acknowledgments
The authors would like to thank Profs. Junxin Liu, He Jizheng and Weidong Wang for their kind help; Xiangchong Liu are gratefully acknowledged for his detailed and patient favor in correlation analysis. This research is financially supported by the National Natural Science Foundation of China (20877086 and 21077119), National Basic Research Program of China (No.2009CB421103), and National Water Project (2008ZX07209-006-02; 2008ZX07421-001; 2009ZX07209-005).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Wang, Y., Feng, X. et al. Quantitative analyses of ammonia-oxidizing Archaea and bacteria in the sediments of four nitrogen-rich wetlands in China. Appl Microbiol Biotechnol 90, 779–787 (2011). https://doi.org/10.1007/s00253-011-3090-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3090-0